Abstract
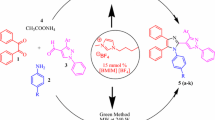
A number of imidazole derivatives 3a–f and 4a–f have been synthesized by the condensation of 3-methylthiophen-2-carboxaldehyde 1a, 5-methylthiophen-2-carboxaldehyde 1b, N-methylpyrrol-2-carboxaldehyde 1c, 1-naphthaldehyde 1d, 2-naphthaldehyde 1e, and 2-hydroxy-1-naphthaldehyde 1f with 1,2-diaminoanthraquinone 2a and 2,3-diaminophenazine 2b, respectively. Condensation of 2-guanidinobenzimidazole with functionalized aldehydes 1a–f leads to the formation of guanidine derivatives 5a–f. Both imidazole (3a–f, 4a–f) and guanidine derivatives (5a–f) were synthesized in good yields using conventional heating and microwave irradiation techniques. Structures assigned to compounds 3a–f, 4a–f and 5a–f are supported by correct spectral and analytic data. On screening for anti-inflammatory and anticancer activities, compounds 3e, 4a and 5a exhibited good anti-inflammatory and compounds 3d, 3f, 4d and 4f showed very good anticancer activity.


Similar content being viewed by others
References
Albrecht W, Hauser D, Laufer S, Striegel H-G, Tollmann K (2008) Imidazole derivatives as antiinflammatory agents and their preparation, pharmaceutical compositions and use in the treatment of inflammation associated with immune system impairment. PCT International Application WO 2008023066, Chem. Abstr. 148, 308343
Bevec D (2004) Pyrimidinium-guanidiniminoethylphenyl-guanidinoalkyl amines and guanidiniminoethylphenyl-guanidinoalkylamines as pharmaceutically active new substances for the treatment of viral infections and other conditions. European patent application EP 1413300, Chem. Abstr. 140, 350626
Brzozowski Z, Saczewski F, Slawinski J (2007) Synthesis of novel 3-amino-2-(4-chloro-2-mercaptobenzenesulfonyl)-guanidine derivatives as potential antitumor agents. Eur J Med Chem 42:1218–1225. doi:10.1016/j.ejmech.2007.01.020
Courtney SM, Hay PA, Scopes DIC (2004) Preparation of benzoxazole, benzothiazole and benzimidazole acid derivatives for the treatment of cancer. PCT International Application WO 2004046122, Chem. Abstr. 141, 7114
Ersan S, Nacak S, Noyanalplan N, Yesilada E (1997) Studies on analgesic and antiinflammatory activities of 1-dialkylaminomethyl-2-(p-substituted phenyl)-5-substituted benzimidazole derivatives. Arzneimittel-Forschung 47:834–836
Gaonkar SL, Lokanatha RKM, Suchetha SN (2009) Microwave-assisted synthesis and evaluation of anti-inflammatory activity of new series of N-substituted 2-butyl-5-chloro-3H-imidazole-4-carbaldehyde derivatives. Med Chem Res 18:221–230. doi:10.1007/s00044-008-9121-4
Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbol EL, Sorensen R, Folke F, Buch P, Gadsboll N, Rasmussen S, Poulsen HE, Kober L, Madsen M, Torp-Pedersen C (2009) Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med 169:141–149
Gupta P, Hameed S, Jain R (2004) Ring-substituted imidazoles as a new class of anti-tuberculosis agents. Eur J Med Chem 39:805–814. doi:10.1016/j.ejmech.2004.05.005
Havaldar FH, Patil AR (2008) Synthesis of biologically active 1-[2-(2-methyl-5-nitroimidazol-1-yl)acetyl]-3-substituted phenyl-4-carbaldehyde-1H-pyrazoles. Asian J Chem 20:97–101
Hensler ME, Bernstein G, Nizet V, Nefzi A (2006) Pyrrolidine bis-cyclic guanidines with antimicrobial activity against drug-resistant Gram-positive pathogens identified from a mixture-based combinatorial library. Bioorg Med Chem Lett 16:5073–5079. doi:10.1016/j.bmcl.2006.07.037
Herrero MA, Kremsner JM, Kappe CO (2008) Nonthermal microwave effects revisited: on the importance of internal temperature monitoring and agitation in microwave chemistry. J Org Chem 73:36–47
Matasova LV, Kryl’skii DV, Popova TN, Makeeva AV, Shikhaliev KS (2008) Cardioprotective properties of benzoxy(thia)azol guanidine derivatives. Farmatsevticheskoi Khimii 56
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766
Oezden S, Atabey D, Yildiz S, Goeker H (2005) Synthesis and potent antimicrobial activity of some novel methyl or ethyl 1H-benzimidazole-5-carboxylates derivatives carrying amide or amidine groups. Bioorg Med Chem 13:1587–1597. doi:10.1016/j.bmc.2004.12.025
Pearce AN, Chia EW, Berridge MV, Maas EW, Page MJ, Harper JL, Webb VL, Copp BR (2008) Orthidines A-E, tubastrine, 3,4-dimethoxyphenethyl-β-guanidine, and 1,14-sperminedihomovanillamide: potential anti-inflammatory alkaloids isolated from the New Zealand ascidian Aplidium orthium that act as inhibitors of neutrophil respiratory burst. Tetrahedron 64:5748–5755. doi:10.1016/j.tet.2008.04.012
Sircar JC, Thomas RJ, Richards ML, Khatuya H (2004) Preparation of imidazole derivatives for treatment of allergic and hyperproliferative disorders. PCT International Application WO 2004091610, Chem. Abstr., 141, 395552
Skehan P, Storeng R, Scudiero D, Monks A, McMohan J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Sondhi SM, Rani R (2008) A convenient, solvent free and high yielding synthesis of bicyclo-heterocyclic compounds. Lett Org Chem 5:51–54. doi:10.2174/157017808783330180
Sondhi SM, Rani R, Gupta PP, Agrawal SK, Saxena AK (2009a) Synthesis, anticancer, and anti-inflammatory activity evaluation of methanesulfonamide and amidine derivatives of 3,4-diaryl-2-imino-4-thiazolines. Mol Divers 13:357–366
Sondhi SM, Rani R, Roy P, Agrawal SK, Saxena AK (2009b) Microwave-assisted synthesis of N-substituted cyclic imides and their evaluation for anticancer and anti-inflammatory activities. Bioorg Med Chem Lett 19:1534–1538. doi:10.1016/j.bmcl.2008.07.048
Sondhi SM, Singh J, Kumar A, Jamal H, Gupta PP (2009c) Synthesis of amidine and amide derivatives and their evaluation for anti-inflammatory and analgesic activities. Eur J Med Chem 44:1010–1015. doi:10.1016/j.ejmech.2008.06.029
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med 111:544–547
Acknowledgements
We thank the technical staff of the Chemistry Department, IIT. Roorkee, for their assistance in spectroscopic studies and elemental analysis. One of the authors—Mr. Jaiveer Singh (SRF-NET)—thanks the CSIR, New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sondhi, S.M., Singh, J., Roy, P. et al. Conventional and microwave-assisted synthesis of imidazole and guanidine derivatives and their biological evaluation. Med Chem Res 20, 887–897 (2011). https://doi.org/10.1007/s00044-010-9410-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9410-6