Abstract
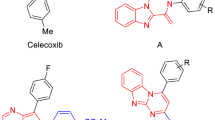
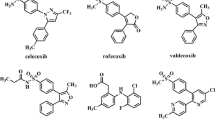
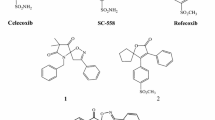
A new series of 2-aryl, 3-benzyl-(1,3-oxazolidine or 1,3-thiazolidine)-4-ones, possessing a methylsulfonyl pharmacophore, were synthesized to evaluate their biological activities as selective cyclooxygenase-2 (COX-2) inhibitors. In vitro COX-1 and COX-2 isozyme inhibition studies were performed to acquire structure-activity relationship data with respect to the point that molecular modeling studies showed that designed compounds bind in the primary binding site such that the C-2 para-SO2Me substituent inserts into the 2° pocket present in COX-2 enzyme. COX-1 and COX-2 inhibition studies showed that all compounds were selective inhibitors of the COX-2 isozyme with IC50 values in the highly potent 0.21 to 0.34 μM range, and COX-2 selectivity indexes in the 222.3 to >476 range. 3-Benzyl-2-(4-methylsulfonylphenyl)-1,3-oxazolidine-4(5H)-one was identified as the most potent (IC50 = 0.21 μM) and selective (S.I. > 476) COX-2 inhibitor among the synthesized compounds. It also was a more selective COX-2 inhibitor than the parent reference compound celecoxib (S.I. > 403).




Similar content being viewed by others
References
Abouzid KW, Bekhit SA (2008) Novel anti-inflammatory agents based on pyridazinone scaffold; design, synthesis and in vivo activity. Bioorg Med Chem 16:5547–5556
Dogné JM, Supuran CT, Pratico D (2005) Cardiovascular effects of the coxibs. J Med Chem 48:2251–2257
Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P (1990) The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 265:16737–16736
Herschman HR (1996) Prostaglandin synthase 2. Biochem Biophys Acta 1299:125–140
Katori M, Majima M (2000) Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm Res 49:367–392
Kawamori T, Rao CV, Seibert K, Reddy BS (1998) Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 58:406–412
Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC (1996) Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 384:644–648
Penning TD, Tally JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers R, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC (1997) Synthesis and biological evaluation of the 1, 5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide (SC-b58635, celecoxib). J Med Chem 40:1347–1365
Prasit P, Wang Z, Brideau C, Chan CC, Charlson S, Cromlish W, Ethier D, Evans JF, Ford-Hutchinson AW, Gauthier JY, Gordon R, Guay J, Gresser M, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini JO, Neil GP, Quellet M, Percival MD, Perrier H, Riendeau D, Rodger I, Tagari P, Therien M, Vikers P, Wong E, Xu L, Young RN, Zamboni R, Boyce S, Rupniak N, Forrest M, Visco D, Patrick D (1999) The discovery of rofecoxib, [MK 966, Vioxx, 4-(4′-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone], an orally active cyclooxygenase-2-inhibitor. Bioorg Med Chem Lett 9:1773–1778
Rammana Reddy MV, Billa VK, Pallela VR, Mallireddigari RB, Boominathan R, Gabriel JL, Reddy EP (2008) Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorg Med Chem 16:3907–3916
Riendeau D, Percival MD, Brideau C, Dube CS, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G, Guay J, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson A, Young RN, Chan CC (2002) Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther 296:558–566
Scholz M, Ulbrich HK, Dannhardt G (2008) Investigations concerning the COX/5-LOX inhibiting and hydroxyl radical scavenging potencies of novel 4, 5-Diaryl isoselenazoles. Eur J Med Chem 43:1152–1159
Smith WL, DeWitt DL (1996) Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62:167–215
Solomon DH (2005) Selective cyclooxygenase 2 inhibitors and. cardiovascular events. Arthritis Rheum 52:1968–1978
Vane JR, Botting RM (1998) Anti-inflammatory drugs and their mechanism of action. Inflamm Res 47:S78–S87
Zarghi A, Rao PNP, Knaus EE (2007a) Synthesis and biological evaluation of methanesulfonamide analogues of rofecoxib: replacement of methanesulfonyl by methanesulfonamide decreases cyclooxygenase-2 selectivity. Bioorg Med Chem 15:1056–1061
Zarghi A, Rao PN, Knaus EE (2007b) Design and synthesis of new rofecoxib analogs as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of the methanesulfonyl pharmacophore by a N-acetylsulfonamido bioisostere. J Pharm Pharmaceut Sci 10:159–167
Zarghi A, Najafnia L, Daraie B, Dadrass OG, Hedayati M (2007c) Synthesis of 2, 3-diaryl-1, 3-thiazolidine-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg Med Chem Lett 17:5634–5637
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarghi, A., Arefi, H., Dadrass, O.G. et al. Design and synthesis of new 2-aryl, 3-benzyl-(1,3-oxazolidine or 1,3-thiazolidine)-4-ones as selective cyclooxygenase (COX-2) inhibitors. Med Chem Res 19, 782–793 (2010). https://doi.org/10.1007/s00044-009-9230-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-009-9230-8