Abstract
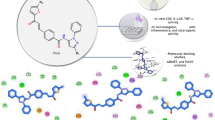
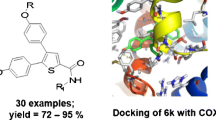
A novel series of 1,3-diarylpyrazole derivatives (4–8), analogues to lonazolac, were designed, synthesized, and evaluated for their anti-inflammatory as well as analgesic activities. To target preferential cyclooxygenase-2 (COX-2) inhibitors, the design of these compounds was based upon two different molecular modeling studies. The first study included fit-comparison study of conformational models of compounds 4–8 with a novel validated COX-2 inhibitors hypothesis generated from the corresponding leads I–V using Hip-Hop CATALYST software. The second study included docking study of the designed compounds 4–8 with binding site of COX-1 and COX-2 enzymes using internal coordinate mechanics (ICM)-Pro software. The reported Akaho method was then used to predict the COX-2 preferentiality of the designed compounds. The designed molecules were synthesized and screened for in vivo anti-inflammatory and analgesic activity. Compounds 4a, 6a, and 8b showed high activity in comparison with indomethacin, consistent with virtual molecular modeling studies.








Similar content being viewed by others
References
Abouzid K, Frohberg P, Lehmann J, Decker M (2007) 6-Aryl-4-oxohexanoic acids: synthesis, effects on eicosanoid biosynthesis, and anti-inflammatory in vivo activities. Med Chem 3:433–436. doi:10.2174/157340607781745393
Akaho E, Fujikawa C, Runion H, Hill C, Nakano H (1999) A study on binding modes of NSAIDs to COX-1 and COX-2 as obtained by Dock 4.0. J Chem Softw 15:1–14. doi:10.2477/jchemsoft.5.1
Bertez G (1987) Inhibiteurs mixtes des voies de la cyclooxygénase et des lipoxygénase, synthése et activté de derives hydrazoniques. Eur J Med Chem 22:147–152. doi:10.1016/0223-5234(87)90010-9
Bratenko MK, Chornous VA, Vovk MV (2001) 4-Functionally substituted 3-heterylpyrazoles: III 3-Aryl(heteryl)pyrazole-4-carboxylic acids and their derivatives. Russ J Org Chem 37:552–555. doi:10.1023/A:1012490120976
Cavasotto CN, Ortiz MA, Abagyanc RA, Piedrafitab FJ (2006) In silico identification of novel EGFR inhibitors with antiproliferative activity against cancer cells. Bioorg Med Chem Lett 16:1969–1974. doi:10.1016/j.bmcl.2005.12.067
Dannhardt G, Kiefer W (2001) Cyclooxygenase inhibitors—current status and future prospects. Eur J Med Chem 36:109–126. doi:10.1016/S0223-5234(01)01197-7
De Luca L, Giacomelli G, Masala S, Porcheddu A (2004) A mild procedure for the preparation of 3-aryl-4-formylpyrazoles. Synlett 13:2299–2302
Habeeb A, Rao P, Knaus E (2001a) Design and synthesis of celecoxib and rofecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of sulfonamide and methylsulfonyl pharmacophores by an azido bioisostere. J Med Chem 44:3039–3042. doi:10.1021/jm010153c
Habeeb AG, Rao PNP, Knaus EE (2001b) Design and synthesis of 4,5-diphenyl-4-isoxazolines: novel inhibitors of cyclooxygenase-2 with analgesic and antiinflammatory activity. J Med Chem 44:2921–2927. doi:10.1021/jm0101287
Hania MM (2005) Synthesis and antibacterial activity of oximes, semicarbazones and phenylhydrazones. Asian J Chem (Kyoto) 17:439–442
Ismail MAH, Aboul-Enein MNY, Abouzid KAM, Seryaa RAT (2006) Ligand design and synthesis of new imidazo[5, 1-B]quinazoline derivatives As A1-adrenoceptor agonists and antagonists. Bioorg Med Chem 14:898–910. doi:10.1016/j.bmc.2005.07.037
Kalgutkar A, Marnett A, Crews B, Remmel R, Marnett L (2000) Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med Chem 43:2860–2870. doi:10.1021/jm000004e
Kalgutkar A, Rowlinson S, Crews B, Marnett L (2002) Amide derivatives of meclofenamic acid as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett 12:521–524. doi:10.1016/S0960-894X(01)00792-2
Kira MA, Abdel-Rahman MO, Gadalla KZ (1969) The Vilsmeier–Haack reaction—III Cyclization of hydrazones to pyrazoles. Tetrahedron Lett 2:109–110. doi:10.1016/S0040-4039(01)88217-4
Kurumbail RG, Stevens AM, Gierse JK, Mcdonald JJ, Stegeman RA, Pak JY (1996) Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 384:644–648. doi:10.1038/384644a0
Li CS, Brideau C, Chan C, Savoie C, Claveau D, Charleson S, Gordon R, Grereig G (2003) Pyridazinone as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett 13:597–600. doi:10.1016/S0960-894X(02)01045-4
Olgen S, Akaho E, Nebioglu D (2001) Synthesis and receptor docking studies of N-substituted indole-2-carboxylic acid esters as a search for COX-2 selective enzyme inhibitors. Eur J Med Chem 36:747–770. doi:10.1016/S0223-5234(01)01258-2
Palomer A, Cabre F, Pascual J, Campos J, Trujillo M, Entrena A, Gallo M, Mauleon D, Espinosa A (2002) Identification of novel cyclooxygenase-2 selective inhibitors using pharmacophore models. J Med Chem 45:1402–1411. doi:10.1021/jm010458r
Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC (1997) Synthesis and biological evaluation of the 1, 5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1 h-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J Med Chem 40:1347–1365. doi:10.1021/jm960803q
Ranatunge RR, Garavey DS, Janero DR, Letts LG, Martino AM, Murty MG, Richardson SK, Young DV, Zemetseva IS (2004) Synthesis and selective cyclooxygenase-2 (COX-2) inhibitory activity of a series of novel bicyclic pyrazoles. Bioorg Med Chem 12:1357–1366. doi:10.1016/j.bmc.2004.01.012
Riedel R (1981) Pharmacological properties of lonazolac-calcium, a new antiinflammatory antirheumatic drug. Arzneim-Forsch Drug Res 31:655–665
Scholz M, Ulbrich HK, Dannhardt G (2008) Investigations concerning the COX/5-LOX inhibiting and hydroxyl radical scavenging potencies of novel 4, 5-diaryl isoselenazoles. Eur J Med Chem 43:1152–1159. doi:10.1016/j.ejmech.2007.09.007
Soliva R, Almansa C, Kalko SG, Luque FJ, Orozco M (2003) Theoretical studies on the inhibition mechanism of cyclooxygenase-2. Is there a unique recognition site? J Med Chem 46:1372–1382. doi:10.1021/jm0209376
Vogel HG (2002) Drug discovery and evaluation: pharmacological assays. Springer, Berlin
Zaheer SH, Hacker IK, Rao NS (1956) The condensation of levulinic acid with aromatic aldehydes. Chem Ber 89:351–354. doi:10.1002/cber.19560890226
Acknowledgment
This work was partially supported by a grant from the Egyptian Ministry of Higher Education and State for Scientific Research (MHESR), ParOwn (0906).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ismail, M.A.H., Lehmann, J., Abou El Ella, D.A. et al. Lonazolac analogues: molecular modeling, synthesis, and in vivo anti-inflammatory activity. Med Chem Res 18, 725–744 (2009). https://doi.org/10.1007/s00044-009-9163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-009-9163-2