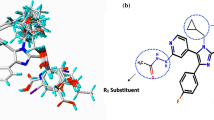
Abstract
Neurodegenerative disorders are consequences of progressive and irreversible loss of neurons due to unwanted apoptosis, which involves caspases, a group of cystein proteases that cleave other proteins and inactivate them. Among several different groups of caspase enzymes, caspases-3 play a key role in apoptosis. Therefore they are used as therapeutic target for their inhibitors. In pursuit of better caspase-3 inhibitors, a quantitative structure–activity relationship analysis was performed on a series of 1,3-dioxo-4-methyl-2, 3-dihydro-1H-pyrrolo [3,4-c] quinolines as caspase-3 inhibitors using WIN CAChe 6.1 and Medicinal Chemistry Regression Machine. The best QSAR model was selected and validated. The values of the statistical data are r = 0.951, F = 65.62, SEE = 0.4175, t = 2.08. The study reveals that, if the partition coefficient (log P), conformational minimum energy (CME), and the lowest unoccupied molecular orbital energy (E LUMO) are altered, the biological activity is increased. On the basis of the selected QSAR model, we designed a new series of compounds, calculated their activity, and found that the compounds were more potent than existing compounds.

Similar content being viewed by others
References
Anselmo DM, Amersi FF, Shen XD, Gao F, Katori M, Lassman C, Ke B, Coito AJ, Ma J, Brinkmann V, Busuttil RW, Kupiec-Weglinski JW, Farmer DG (2002) FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant 2:843–849
Chapman JG, Magee WP, Stukenbrok HA, Beckius GE, Milici AJ, Tracey WR (2002) A novel nonpeptide caspase-3/7 inhibitor, (S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin reduces myocardial ischemic injury. Eur J Pharmacol 456:59–68
Han BH, Xu D, Choi J, Han Y, Xanthoudakis S, Roy S, Tam J, Vaillancourt J, Colucci J, Siman R, Giroux A, Robertson GS, Zamboni R, Nicholson DW, Holtzman DM (2002) Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J Biol Chem 277:30128–30136
Haunstetter A, Izumo S (1998) Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res 82:1111–1129
Hoglen NC, Chen LS, Fisher CD, Hirakawa BP, Groessl T, Contreras PC (2004) Characterization of IDN-6556 (3-[2-(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino]-4-oxo-5-(2,3,5,6-tetrafluoro- phenoxy)- pentanoic acid): a liver-targeted caspase inhibitor. J Pharmacol Exp Ther 309:634–640
Jacobson MD (1998) Anti-apoptosis therapy: a way of treating neural degeneration. Curr Biol 8:418–421
Kravchenko DV, Kysil VV, Ilyn AP, Tkachenko SE, Maliarchouk S, Okun IM, Ivachtchenko AV (2005) 1,3-Dioxo-4-methyl-2,3-dihydro-1H-pyrrolo[3,4-c]quinolines as potent caspase-3 inhibitors. Bioorg Med Chem Lett 15:1841–1845
Marques CA, Keil U, Bonert A, Steiner B, Haass C, Muller WE, Eckert A (2003) Neurotoxic mechanisms caused by the Alzheimer’s disease-linked Swedish amyloid precursor protein mutation: oxidative stress, caspases, and the JNK pathway. J Biol Chem 278:28294–28302
Mohr A, Zwacka RM (2007) In situ trapping of initiator caspases reveals intermediate surprises. Cell Biol Int 31:526–530
Parrill AL (2003) HIV-1 integrase inhibition: binding sites, structure acti vityrelationships and future perspectives. Curr Med Chem 10:1811–1824
Scott CW, Sobotka-Briner C, Wilkins DE, Jacobs RT, Folmer JJ, Frazee WJ, Bhat RV, Ghanekar SV, Aharony D (2003) Novel small molecule inhibitors of caspase-3 block cellular and biochemical features of apoptosis. J Pharmacol Exp Ther 304:433–440
Wang QM, Johnson RB, Jungheim LN, Cohen JD, Villarreal EC (1998) Dualinhibition of human rhinovirus 2A and 3C proteases by homophthalimides. Antimicrob Agents Chemother 42:916–920
Webber SE, Okano K, Little TL, Reich SH, Xin Y, Fuhrman SA, Matthews DA, Love RA, Hendrickson TF, Patick AK, Meador JW, Ferre RA, Brown EL, Ford CE, Binford SL, Worland ST (1998) Tripeptide aldehyde inhibitors of human rhinovirus 3C protease: design, synthesis, biological evaluation, and cocrystal structure solution of P1 glutamine isosteric replacements. J Med Chem 41:2786–2805
Webber SE, Tikhe J, Worland ST, Fuhrman SA, Hendrickson TF, Matthews DA, Love RA, Patick AK, Meador JW, Ferre RA, Brown EL, DeLisle DA, Ford CE, Binford SL (1996) Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J Med Chem 39:5072–5082
Wellington C, Hayden M (2000) Caspases and neurodegeneration: on the cutting edge of new therapeutic approaches. Clin Genet 57:1–10
Acknowledgement
One of the authors Mr. Simant Sharma is grateful to U.G.C. for providing a fellowship for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S., Sahu, K., Jain, P. et al. QSAR study of 1,3–dioxo-4-methyl-2,3-dihydro-1h-pyrrolo[3,4-c]quinolines as caspase-3 inhibitors. Med Chem Res 17, 399–411 (2008). https://doi.org/10.1007/s00044-007-9075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-007-9075-y