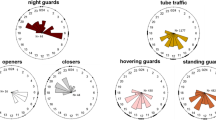
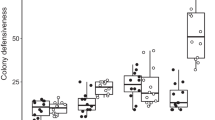
Abstract
Bees may leave their nest in the event of an attack, but this is not their only response. Here, we examine the behavior of those individuals that remain inside the nest during a disturbance. Specifically, we test the hypothesis that bee workers usually exhibiting high levels of inactivity (i.e., ‘lazy’ bees) may function as defensive reserves that are more likely to respond when the colony is disturbed. We explore this hypothesis by simulating vertebrate attacks by vibrating or blowing carbon dioxide into two colonies on alternating days and measuring the movements and tasks performed by bees inside the nest. Our results show that regardless of the disturbance type, workers increase guarding behavior after a disturbance stops. Although previously inactive bees increased their movement speed inside the nest when the disturbance was vibration, they were not more likely to leave the nest (presumably to attack the simulated attacker) or switch to guarding behavior for any disturbance type. We therefore reject the hypothesis that inactive Bombus impatiens bumblebees act as defensive reserves, and propose alternative hypotheses regarding why many workers remain inactive inside the nest.




Similar content being viewed by others
References
Baglione V., Canestrari D., Chiarati E., Vera R. and Marcos J.M. 2010. Lazy group members are substitute helpers in carrion crows. Proc. R. Soc. B-Biol. Sci. 277: 3275–3282
Breed M.D., Robinson G.E. and Page R.E. 1990. Division of labor during honey bee colony defense. Behav. Ecol. Sociobiol. 27: 395–401
Brown S.G., Boettner G.H. and Yack J.E. 2007. Clicking caterpillars: acoustic aposematism in Antheraea polyphemus and other Bombycoidea. J. Exp. Biol. 210: 993–1005
Bruschini C., Cervo R. and Turillazzi S. 2005. Defensive responses to visual and vibrational stimulations in colonies of the social wasp Polistes dominulus. Ethol. Ecol. Evol. 17: 319–326
Bura V.L., Fleming A.J. and Yack J.E. 2009. What’s the buzz? Ultrasonic and sonic warning signals in caterpillars of the great peacock moth (Saturnia pyri). Naturwissenschaften 96: 713–718
Cameron S.A. 1989. Temporal patterns of division of labor among workers in the primitively eusocial bumble bee, Bombus griseocollis (Hymenoptera: Apidae). Ethology 80: 137–151
Cole B.J. 1986. The social behavior of Leptothorax allardycei (Hymenoptera, Formicidae): time budgets and the evolution of worker reproduction. Behav. Ecol. Sociobiol. 18: 165–173
Collins A.M., Rinderer T.E., Daly H.V., Harbo J.R. and Pesante D. 1989. Alarm pheromone production by two honeybee (Apis mellifera) types. J. Chem. Ecol. 15: 1747–1756
Couvillon M.J., Robinson E.J.H., Atkinson B., Child L., Dent K.R. and Ratnieks F.L.W. 2008. En garde: rapid shifts in honeybee, Apis mellifera, guarding behaviour are triggered by onslaught of conspecific intruders. Anim. Behav. 76: 1653–1658
Cronin A.L. and Field J. 2007. Rank and colony defense against conspecifics in a facultatively eusocial hover wasp. Behav. Ecol. 18: 331–336
Dornhaus A., Holley J.A., Pook V.G., Worswick G. and Franks N.R. 2008. Why do not all workers work? Colony size and workload during emigrations in the ant Temnothorax albipennis. Behav. Ecol. Sociobiol. 63: 43–51
Evans E., Burns I. and Spivak M. 2007. Befriending Bumble Bees: A Practical Guide to Raising Local Bumble Bees. Regents of the University of Minnesota, Minneapolis
Evans T.A. 2006. Foraging and building in subterranean termites: task switchers or reserve labourers? Insect. Soc. 53: 56–64
Fletcher D.J.C. 1978. The African bee, Apis mellifera adansonii, in Africa. Annu. Rev. Entomol. 23: 151–171
Fletcher L.E. 2007. Vibrational signals in a gregarious sawfly larva (Perga affinis): group coordination or competitive signaling? Behav. Ecol. Sociobiol. 61: 1809–1821
Gordon D.M. 1989. Dynamics of task switching in harvester ants. Anim. Behav. 38: 194–204
Heinrich B. 2004. Bumblebee Economics. Harvard University Press, Cambridge, MA
Herbers J.M. 1983. Social organization in Leptothorax ants: within and between species patterns. Psyche 90: 361–386
Jandt J.M. and Dornhaus A. 2009. Spatial organization and division of labour in the bumblebee Bombus impatiens. Anim. Behav. 77: 641–651
Jandt J.M. and Dornhaus A. 2011. Competition and cooperation: bumblebee spatial organization and division of labor may affect worker reproduction late in life. Behav. Ecol. Sociobiol. 65: 2341–2349
Jandt J.M., Huang E. and Dornhaus A. 2009. Weak specialization of workers inside a bumble bee (Bombus impatiens) nest. Behav. Ecol. Sociobiol. 63: 1829–1836
Jeanne R.L. 1981. Alarm recruitment, attack behavior, and the role of the alarm pheromone in Polybia occidentalis (Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 9: 143–148
Jeanne R.L., Williams N.M. and Yandell B.S. 1992. Age polyethism and defense in a tropical social wasp (Hymenoptera: Vespidae). J. Insect Behav. 5: 211–227
Johnson B.R. 2002. Reallocation of labor in honeybee colonies during heat stress: the relative roles of task switching and the activation of reserve labor. Behav. Ecol. Sociobiol. 51: 188–196
Judd T.M. 2000. Division of labour in colony defence against vertebrate predators by the social wasp Polistes fuscatus. Anim. Behav. 60: 55–61
Kirchner W.H. and Röschard J. 1999. Hissing in bumblebees: an interspecific defence signal. Insect. Soc. 46: 239–243
Klein B.A., Olzsowy K.M., Klein A., Saunders K.M. and Seeley T.D. 2008. Caste-dependent sleep of worker honey bees. J. Exp. Biol. 211: 3028–3040
Korb J. and Schmidinger S. 2004. Help or disperse? Cooperation in termites influenced by food conditions. Behav. Ecol. Sociobiol. 56: 89–95
Lindauer M. 1952. Ein Beitrag zur Frage der Arbeitsteilung im Bienenstaat. Z. Vergl. Physiol. 34: 299–345
Michener C.D. 1964. Reproductive efficiency in relation to colony size in Hymenopterous societies. Insect. Soc. 11: 317–342
O’Donnell S. and Foster R.L. 2001. Thresholds of response in nest thermoregulation by worker bumble bees, Bombus bifarius nearcticus (Hymenoptera: Apidae). Ethology 107: 387–399
O’Donnell S. and Jeanne R.L. 2002. The nest as fortress: defensive behavior of Polybia emaciata, a mud-nesting eusocial wasp. J. Insect Sci. 2: 1–5
Ramirez S. and Cameron S.A. 2003. Army ant attacks by Eciton hamatum and E. rapax (Hymenoptera: Formicidae) on nests of the Amazonian bumble bee, Bombus transversalis (Hymenoptera: Apidae). J. Kans. Entomol. Soc. 76: 533–535
Rohrig A., Kirchner W.H. and Leuthold R.H. 1999. Vibrational alarm communication in the African fungus-growing termite genus Macrotermes (Isoptera, Termitidae). Insect. Soc. 46: 71–77
Schmid-Hempel P. 1990. Reproductive competition and the evolution of work load in social insects. 135: 501–526
Sen Sarma M., Fuchs S., Werber C. and Tautz R. 2002. Worker piping triggers hissing for coordinated colony defence in the dwarf honeybee Apis florea. Zoology 105: 215–223
Tindo M. and Dejean A. 2000. Dominance hierarchy in colonies of Belonogaster juncea juncea (Vespidae, Polistinae). Insect. Soc. 47: 158–163
Visscher P.K. and Vetter R.S. 1995. Smoke and target color effects on defensive behavior in yellowjacket wasps and bumble bees (Hymenoptera: Vespidae, Apidae) with a description of an electronic attack monitor. J. Econ. Entomol. 88: 579–583
Weidenmüller A. 2004. The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav. Ecol. 15: 120–128
Weidenmüller A., Kleineidam C. and Tautz J. 2002. Collective control of nest climate parameters in bumblebee colonies. Anim. Behav. 63: 1065–1071
Acknowledgments
We are grateful to Diane Simon in her assistance in data collection. Judie Bronstein, Dan Papaj, John Pepper, Diana Wheeler, Maggie Couvillon, and Anna Himler provided feedback on the manuscript. Research supported through the College of Science, Department of Ecology and Evolutionary Biology, University of Arizona, and NSF grant to AD (grant no. IOS 0841756).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jandt, J.M., Robins, N.S., Moore, R.E. et al. Individual bumblebees vary in response to disturbance: a test of the defensive reserve hypothesis. Insect. Soc. 59, 313–321 (2012). https://doi.org/10.1007/s00040-012-0222-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-012-0222-1