Abstract
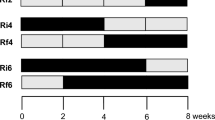
Flow pulses that alternately immerse and expose benthic habitats are widely recognized as key determinants of biodiversity and ecosystem functioning in rivers. Terrestrial leaf litter input, colonization, and breakdown are also key processes in river ecosystems, but little is known about the effects of alternating immersion and emersion on these processes. We used litterbags to examine breakdown, microbial activity, and colonization of Populus sp. leaves by invertebrates along a natural gradient in immersion and emersion (i.e., submergence and exposure to air) in a temporary river. Rates of leaf litter mass loss, microbial activity and colonization by invertebrates differed among litterbags that were permanently immersed, intermittently immersed and permanently emersed, and breakdown rate coefficients (k) decreased with increasing cumulative emersed duration (the total number of day of emersion during the experiment). In contrast, the frequency of emersed periods had no detectable effects on these variables. k was positively correlated with the density of invertebrate shredders in immersed litterbags, with microbial activity and shredder density in intermittent litterbags, and with microbial activity in emersed litterbags. These correlations suggest that the relative importance of microbial activity on k increases with emersed duration, due to the periodic elimination of aquatic shredders and the scarcity of terrestrial detritivores. The fact that leaf litter breakdown was detectable under permanently emersed conditions indicates that mechanisms other than shredding by invertebrates, such as leaching and photodegradation, are dominant in dry river habitats.








Similar content being viewed by others
References
Abelho M (2008) Effects of leaf litter species on macroinvertebrate colonization during decomposition in a Portuguese stream. Int Rev Hydrobiol 93:358–371
Amalfitano S, Fazi S, Zoppini A, Barra-Caracciolo A, Grenni P, Puddu A (2008) Responses of benthic bacteria to experimental drying in sediments from Mediterranean temporary rivers. Microb Ecol 55:270–279
Andersen DC, Nelson SM (2006) Flood pattern and weather determine Populus leaf litter breakdown and nitrogen dynamics on a cold desert floodplain. J Arid Environ 64:626–650
Arscott DB, Larned S, Scarsbrook MR, Lambert P (2010) Aquatic invertebrate community structure along an intermittence gradient: Selwyn River, New Zealand. J North Am Benthol Soc 29:530–545
Arsuffi TL, Suberkropp K (1988) Effects of fungal mycelia and enzymatically degraded leaves on feeding and performance of caddisfly (Trichoptera) larvae. J North Am Benthol Soc 7:205–211
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Baldy V, Gessner MO, Chauvet E (1995) Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos 74:93–102
Bärlocher F (2007) Decomposition and fungal community structure in aquatic environments. In: Hurst CJ, Crawford RL, Garland JL, Lipson DA, Mills AL, Stetzenbach LD (eds) Manual of environmental microbiology, 3rd edn. ASM Press, Washington, pp 469–478
Battle JM, Golladay SW (2001) Hydroperiod influence on breakdown of leaf litter in cypress-gum wetlands. Am Midl Nat 146:128–145
Benke AC, Chaubey I, Ward M, Dunn EL (2000) Flood pulse dynamics of an unregulated river floodplain in the Southeastern U.S. coastal plain. Ecology 81:2730–2741
Benstead JP, March JG, Pringle CM, Ewel KC, Short JW (2009) Biodiversity and ecosystem function in species-poor communities: community structure and leaf litter breakdown in a pacific island stream. J North Am Benthol Soc 28:454–465
Boulton AJ (1991) Eucalypt leaf decomposition in an intermittent stream in south-eastern Australia. Hydrobiol 211:123–136
Buffington JM, Lisle TE, Woodsmith RD, Hilton S (2002) Controls on the size and occurrence of pools in coarse-grained forest rivers. River Res Appl 18:507–531
Chadderton WL (1988) Faunal and chemical characteristics of some Stewart Island streams. N Z Nai Sci 15:43–50
Chergui H, Pattee E (1991) An experimental study of the breakdown of submerged leaves by hyphomycetes and invertebrates in Morocco. Freshw Biol 26:97–110
Collins SL, Sinsabaugh RL, Crenshaw C, Green L, Porras-Alfaro A, Stursova M, Zeglin LH (2008) Pulse dynamics and microbial processes in aridland ecosystems. J Ecol 96:413–420
Datry T, Larned ST (2008) River flow controls ecological processes and invertebrate assemblages in subsurface flowpaths of an ephemeral river reach. Can J Fish Aquat Sci 65:1532–1544
Datry T, Larned ST, Scarsbrook MR (2007) Responses of hyporheic invertebrate assemblages to large-scale variation in flow permanence and surface-subsurface exchange. Freshw Biol 52:1452–1462
Datry T, Corti R, Claret C, Philippe M (2011) Flow intermittence controls leaf litter decomposition in a French temporary alluvial river: the “drying memory”. Aquat Sci. doi:10.1007/s00027-011-0193-8
Fontvieille D, Outaguerouine A, Thevenot DR (1992) Fluorescein diacetate hydrolysis as a measure of microbial activity in aquatic systems: application to activated sludges. Environ Technol 13:531–540
Gallo ME, Porras-Alfaro A, Odenbach KJ, Sinsabaugh RL (2009) Photoacceleration of plant litter decomposition in an arid environment. Soil Biol Biochem 41:1433–1441
Gaudes A, Artigas J, Romani AM, Sabater S, Munoz I (2009) Contribution of microbial and invertebrate communities to leaf litter colonization in a Mediterranean stream. J North Am Benthol Soc 28:34–43
Gonçalves JF, Graça MAS, Callisto M (2007) Litter decomposition in a Cerrado savannah stream is retarded by leaf toughness, low dissolved nutrients and a low density of shredders. Freshw Biol 52:1440–1451
Greenwood MJ, McIntosh AR (2008) Flooding impacts on responses of a riparian consumer to cross-ecosystem subsidies. Ecology 89:1489–1496
Greenwood MJ, McIntosh AR (2010) Low river flow alters the biomass and population structure of a riparian predatory invertebrate. Freshw Biol 55:2062–2076
Hutchens JJ, Wallace JB (2002) Ecosystem linkages between southern Appalachian headwater streams and their banks: leaf litter breakdown and invertebrate assemblages. Ecosystems 5:80–91
Iovieno P, Bääth E (2008) Effect of drying and rewetting on bacterial growth rates in soil. FEMS Microb Ecol 65:400–407
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of the International Large River Symposium, vol 106. Canadian Special Publication of Fisheries and Aquatic Sciences, pp 110–127
Kirby JM, Webster JR, Benfield EF (1983) The role of shredders in detrital dynamics of permanent and temporary streams. In: Fontaine TD, Bartell SM (eds) Dynamics of lotic ecosystems. Ann Arbor, USA, pp 425–435
Kuehn KA, Steiner D, Gessner MO (2004) Diel mineralization patterns of standing-dead plant litter: implications for CO2 flux from wetlands. Ecology 85:2504–2518
Langhans SD, Tockner K (2006) The role of timing, duration, and frequency of inundation in controlling leaf-litter and in a river-floodplain ecosystem (Tagliamento, NE Italy). Oecologia 147:501–509
Langhans SD, Tiegs SD, Gessner M, Tockner K (2008) Leaf decomposition heterogeneity across a riverine floodplain mosaic. Aquat Sci 70:337–346
Larned ST, Hicks MD, Schmidt J, Davey AJH, Dey K, Scarsbrook M, Arscott DB, Woods RA (2008) The Selwyn River of New Zealand: a benchmark system for alluvial plain rivers. River Res Appl 24:1–21
Larned ST, Datry T, Arscott DB, Tockner K (2010a) Emerging concepts in temporary-river ecology. Freshw Biol 55:717–738
Larned ST, Schmidt J, Datry T, Konrad CP, Dumas JK, Diettrich JC (2010b) Longitudinal river ecohydrology: flow variation down the lengths of alluvial rivers. Ecohydrology. doi:10.1002/eco.126
Ligeiro R, Moretti MS, Gonçalves JF, Callisto M (2010) What is more important for invertebrate colonization in a stream with low-quality litter inputs: exposure time or leaf species ? Hydrobiology 654:125–136
Linklater W (1995) Breakdown and detritivore colonization of leaves in three New Zealand streams. Hydrobiology 306:241–250
Maamri A, Chergui H, Pattee E (1997) Leaf litter processing in a temporary northeasten Moroccan river. Arch Hydrobiol 140:513–531
McKenzie R, Conner B, Bodeker G (1999) Increased summertime UV radiation in New Zealand in response to ozone loss. Science 285:1709–1711
Neatrour MA, Webster JR, Benfield EF (2004) The role of floods in particulate organic matter dynamics of a southern Appalachian river-floodplain ecosystem. J North Am Benthol Soc 23:198–213
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic-terrestrial linkages along a braided river: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759
Paetzold A, Yoshimura C, Tockner K (2008) Riparian arthropod responses to flow regulation and river channelisation. J Appl Ecol 45:894–903
Petersen RC, Cummins KW (1974) Leaf processing in a woodland stream. Freshw Biol 4:343–368
Reinfields I, Nanson G (1993) Formation of braided river floodplains, Waimakariri River, New Zealand. Sedimentology 40:1113–1127
Richardson JS (1992) Food, microhabitat, or both? Macroinvertebrate use of leaf accumulations in a montane stream. Freshw Biol 27:169–176
Rounick JS, Winterbourn MJ (1983) Leaf processing in two contrasting beech forest streams: effects of physical and biotic factors on litter breakdown. Arch Hydrobiol 96:448–474
Rupp DE, Larned ST, Arscott DB, Schmidt J (2008) Reconstruction of a daily flow record along a hydrologically complex alluvial river. J Hydrol 359:88–104
Ryder DS, Horwitz P (1995) Seasonal water regimes and leaf litter processing in a wetland on the Swan Coastal Plain, Western Australia. Mar Freshw Res 46:1077–1084
Stanley EH, Fisher SG, Grimm NB (1997) Ecosystem expansion and contraction in streams. Bioscience 47:427–435
Steward AL, Bunn SE, Tockner K, Sheldon F, Choy S (2011) Colonization and use of dry stream beds by terrestrial invertebrates. Aquat Sci (this issue)
Swift MJ, Heal OW, Anderson JM (1979). Decomposition in terrestrial ecosystems. In: Studies of ecology, vol 5. University of California Press, Berkeley
Takeda H (1995) A 5 year study of litter decomposition processes in a Chamaecyparis obtusa. Endl For Ecol Res 10:95–104
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J North Am Benthol Soc. 29:118–146
Taylor CB, Wilson DD, Brown LJ, Stewart MK, Burden RJ, Brailsford GW (1989) Sources and flow of North Canterbury Plains groundwater, New Zealand. J Hydrol 106:311–340
Tockner K, Malard F, Ward JV (2000) An extension of the flood pulse concept. Hydrol Process 14:2861–2883
Uetz GW, van der Lahn KL, Summers KL, Gibson PAK, Getz LL (1979) The effects of flooding on floodplain arthropod distribution, abundance and community structure. Am Midl Nat 101:289–299
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104
Ward JV, Tockner K, Arscott DB, Claret C (2002) Riverine landscape diversity. Freshw Biol 47:517–539
Webster JR, Benfield EF, Hutchens JJ, Tank JL, Golladay SW, Adams JC (2001) Do leaf breakdown rates actually measure leaf disappearance from streams? Int Rev Hydrobiol 86:417–427
Wishart MJ (2000) The terrestrial invertebrate fauna of a temporary stream in southern Africa. Afr Zool 35:193–200
Acknowledgments
We thank Helena Campbell, Leonie Clitherow, Christopher Dilley and David Thomas for assistance with field and laboratory work, Jochen Bind for help with elevation profiles, and Jon Harding for help with invertebrate classification. We thank Nicolas Lamouroux, Pierre Marmonnier and two anonymous reviewers for comments that improved the manuscript. Research funding was provided by the New Zealand Foundation for Research Science and Technology, Water Allocation Program (Contract C01X0308), and by Cemagref.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the Special Issue “Recent Perspectives on Temporary River Ecology”.
Rights and permissions
About this article
Cite this article
Corti, R., Datry, T., Drummond, L. et al. Natural variation in immersion and emersion affects breakdown and invertebrate colonization of leaf litter in a temporary river. Aquat Sci 73, 537–550 (2011). https://doi.org/10.1007/s00027-011-0216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-011-0216-5