Abstract.
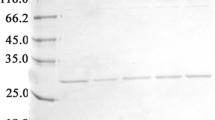
Each kringle of human plasminogen (HPg) except kringle 3 (K3) exhibits affinity for ω-aminocarboxylic acids. Assuming that the K3 domain contains a preformed but nonfunctional lysine binding site (LBS), Lys311 was altered by site-directed mutagenesis into Asp311 in accordance with the consensus sequence of the LBS. Cys297 involved in the interkringle disulfide bridge was mutated into Ser297 to minimize dimerization and aggregation. The mutated K3 TYQ[K3HPg/C297S/ K311D]DS (r-K3mut) was expressed in Escherichia coli, isolated on an Ni2+-nitrilotriacetic acid-agarose column, refolded and purified on a lysine Bio-Gel column. Fluorescence titration indicates affinity of r-K3mut for ω-aminocarboxylic acids with the following association constants (Kass, mM−1) 5-aminopentanoic acid 1.3; 6-aminohexanoic acid 4.2; 7-aminoheptanoic acid 0.5; trans-(aminomethyl)cyclohexanecarboxylic acid 12.7; p-benzylaminesulfonic acid 11.8. r-K3mut exhibits an affinity similar to native and mutated (R220G, E221D) K2. The results indicate the presence of a preformed but nonfunctional LBS in native K3 of HPg. We were able to demonstrate for the first time that an appropriate mutation in the LBS of a kringle produced a weak but distinct affinity for ω-aminocarboxylic acids.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 17 November 1998; accepted 19 November 1998
Rights and permissions
About this article
Cite this article
Bürgin, J., Schaller, J. Expression, isolation and characterization of a mutated human plasminogen kringle 3 with a functional lysine binding site. CMLS, Cell. Mol. Life Sci. 55, 135–141 (1999). https://doi.org/10.1007/s000180050278
Published:
Issue Date:
DOI: https://doi.org/10.1007/s000180050278