Abstract.
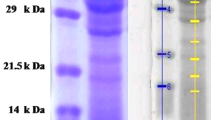
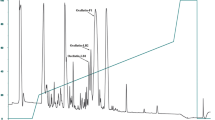
Three antibacterial proteins were isolated from acid extracts of channel catfish (Ictalurus punctatus) skin by cation exchange chromatography and reverse-phase high-pressure liquid chromatography. The molecular masses of the proteins were 15.5, 15.5 and 30 kD as determined by SDS-polyacrylamide gel electrophoresis. Mass spectrometry, amino acid composition and amino acid sequence data suggest that the most abundant protein is closely related to histone H2B. The H2B-like protein was inhibitory to Aeromonas hydrophila and Saprolegnia spp., which are important bacterial and fungal pathogens of fish. These findings suggest that histones may be important defensive molecules in fish.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 22 December 1997; received after revision 5 March 1998; accepted 5 March 1998
Rights and permissions
About this article
Cite this article
Robinette, D., Wada, S., Arroll, T. et al. Antimicrobial activity in the skin of the channel catfish Ictalurus punctatus: characterization of broad-spectrum histone-like antimicrobial proteins. CMLS, Cell. Mol. Life Sci. 54, 467–475 (1998). https://doi.org/10.1007/s000180050175
Issue Date:
DOI: https://doi.org/10.1007/s000180050175