Abstract.
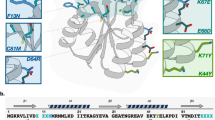
The selection of novel proteins or enzymes from random protein libraries has come to be a major objective in current biology, and these enzymes should prove useful in various biological and biomedical fields. New technologies such as in vitro selection of proteins in cell-free systems have high potential to realize evolu tionary molecular engineering of proteins. This review highlights an application of insertional mutagenesis of proteins to evolutionary molecular engineering. Random sequence proteins are inserted into the surface of a host enzyme which serves as a scaffold to display random protein libraries. Constraints on random polypeptide conformations owing to the proximity of N- and C-termini on the scaffold would result in greater screening efficiency of libraries. The scaffold enzyme is also used as a probe for monitoring the hill climbing of random sequence proteins on a fitness landscape and navigating rapid protein folding in the sequence space.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 9 October 1997; received after revision 6 January 1998; accepted 19 January 1998
Rights and permissions
About this article
Cite this article
Doi, N., Yanagawa, H. Screening of conformationally constrained random polypeptide libraries displayed on a protein scaffold. CMLS, Cell. Mol. Life Sci. 54, 394–404 (1998). https://doi.org/10.1007/s000180050169
Issue Date:
DOI: https://doi.org/10.1007/s000180050169