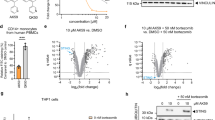
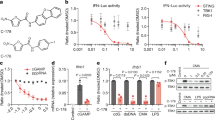
Abstract
STING acts as a cytosolic nucleotide sensor to trigger host defense upon viral or bacterial infection. While STING hyperactivation can exert anti-tumor effects by increasing T cell filtrates, in other contexts hyperactivation of STING can contribute to autoimmune and neuroinflammatory diseases. Several STING targeting agonists and a smaller subset of antagonists have been developed, yet STING targeted degraders, or PROTACs, remain largely underexplored. Here, we report a series of STING-agonist derived PROTACs that promote STING degradation in renal cell carcinoma (RCC) cells. We show that our STING PROTACs activate STING and target activated/phospho-STING for degradation. Locking STING on the endoplasmic reticulum via site-directed mutagenesis disables STING translocation to the proteasome and resultingly blocks STING degradation. We also demonstrate that PROTAC treatment blocks downstream innate immune signaling events and attenuates the anti-viral response. Interestingly, we find that VHL acts as a bona fide E3 ligase for STING in RCC; thus, VHL-recruiting STING PROTACs further promote VHL-dependent STING degradation. Our study reveals the design and biological assessment of VHL-recruiting agonist-derived STING PROTACs, as well as demonstrates an example of hijacking a physiological E3 ligase to enhance target protein degradation via distinct mechanisms.






Similar content being viewed by others
Data and materials availability
All data supporting the findings in this study are available from the corresponding author upon reasonable request.
References
Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE (2013) The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3(5):1355–1361
Ishikawa H, Ma Z, Barber GN (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461(7265):788–792
Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, Yamamoto N, Kawai T, Ishii K, Takeuchi O, Yoshimori T, Akira S (2009) Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci USA 106(49):20842–20846
Xiao TS, Fitzgerald KA (2013) The cGAS-STING pathway for DNA sensing. Mol Cell 51(2):135–139
Corrales L, McWhirter SM, Dubensky TW Jr, Gajewski TF (2016) The host STING pathway at the interface of cancer and immunity. J Clin Invest 126(7):2404–2411
Holm CK, Rahbek SH, Gad HH, Bak RO, Jakobsen MR, Jiang Z, Hansen AL, Jensen SK, Sun C, Thomsen MK, Laustsen A, Nielsen CG, Severinsen K, Xiong Y, Burdette DL, Hornung V, Lebbink RJ, Duch M, Fitzgerald KA, Bahrami S, Mikkelsen JG, Hartmann R, Paludan SR (2016) Influenza A virus targets a cGAS-independent STING pathway that controls enveloped RNA viruses. Nat Commun 7:10680
Ishikawa H, Barber GN (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455(7213):674–678
Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB (2008) The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29(4):538–550
Wu J, Dobbs N, Yang K, Yan N (2020) Interferon-independent activities of mammalian sting mediate antiviral response and tumor immune evasion. Immunity 53(1):115-126 e5
Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A (2011) Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio 2(3):e00016-11
Archer KA, Durack J, Portnoy DA (2014) STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog 10(1):e1003861
Konno H, Yamauchi S, Berglund A, Putney RM, Mule JJ, Barber GN (2018) Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 37(15):2037–2051
Falahat R, Berglund A, Putney RM, Perez-Villarroel P, Aoyama S, Pilon-Thomas S, Barber GN, Mule JJ (2021) Epigenetic reprogramming of tumor cell-intrinsic STING function sculpts antigenicity and T cell recognition of melanoma. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2013598118
Decout A, Katz JD, Venkatraman S, Ablasser A (2021) The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol 21(9):548–569
Warner JD, Irizarry-Caro RA, Bennion BG, Ai TL, Smith AM, Miner CA, Sakai T, Gonugunta VK, Wu J, Platt DJ, Yan N, Miner JJ (2017) STING-associated vasculopathy develops independently of IRF3 in mice. J Exp Med 214(11):3279–3292
Wu J, Chen YJ, Dobbs N, Sakai T, Liou J, Miner JJ, Yan N (2019) STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med 216(4):867–883
Zhang M, Zou Y, Zhou X, Zhou J (2022) Inhibitory targeting cGAS-STING-TBK1 axis: Emerging strategies for autoimmune diseases therapy. Front Immunol 13:954129
Zhao J, Xiao R, Zeng R, He E, Zhang A (2022) Small molecules targeting cGAS-STING pathway for autoimmune disease. Eur J Med Chem 238:114480
Prabakaran T, Troldborg A, Kumpunya S, Alee I, Marinkovic E, Windross SJ, Nandakumar R, Narita R, Zhang BC, Carstensen M, Vejvisithsakul P, Marqvorsen MHS, Iversen MB, Holm CK, Ostergaard LJ, Pedersen FS, Pisitkun T, Behrendt R, Pisitkun P, Paludan SR (2021) A STING antagonist modulating the interaction with STIM1 blocks ER-to-Golgi trafficking and inhibits lupus pathology. EBioMedicine 66:103314
Liu J, Yuan L, Ruan Y, Deng B, Yang Z, Ren Y, Li L, Liu T, Zhao H, Mai R, Chen J (2022) Novel CRBN-recruiting proteolysis-targeting chimeras as degraders of stimulator of interferon genes with in vivo anti-inflammatory efficacy. J Med Chem 65(9):6593–6611
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ (2001) Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA 98(15):8554–8559
Gooding S, Ansari-Pour N, Towfic F, Ortiz Estevez M, Chamberlain PP, Tsai KT, Flynt E, Hirst M, Rozelle D, Dhiman P, Neri P, Ramasamy K, Bahlis N, Vyas P, Thakurta A (2021) Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood 137(2):232–237
Barrio S, Munawar U, Zhu YX, Giesen N, Shi CX, Via MD, Sanchez R, Bruins L, Demler T, Muller N, Haertle L, Garitano A, Steinbrunn T, Danhof S, Cuenca I, Barrio-Garcia C, Braggio E, Rosenwald A, Martinez-Lopez J, Rasche L, Raab MS, Stewart AK, Einsele H, Stuhmer T, Kortum KM (2020) IKZF1/3 and CRL4(CRBN) E3 ubiquitin ligase mutations and resistance to immunomodulatory drugs in multiple myeloma. Haematologica 105(5):e237–e241
Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, Tran JL, Moore P, Lehmann S, Eberl HC, Muelbaier M, Schneck JL, Clemens J, Adam M, Mehlmann J, Romano J, Morales A, Kang J, Leister L, Graybill TL, Charnley AK, Ye G, Nevins N, Behnia K, Wolf AI, Kasparcova V, Nurse K, Wang L, Puhl AC, Li Y, Klein M, Hopson CB, Guss J, Bantscheff M, Bergamini G, Reilly MA, Lian Y, Duffy KJ, Adams J, Foley KP, Gough PJ, Marquis RW, Smothers J, Hoos A, Bertin J (2018) Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564(7736):439–443
Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, Jorgensen WL, Ciulli A, Crews CM (2012) Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J Am Chem Soc 134(10):4465–4468
Ergun SL, Fernandez D, Weiss TM, Li L (2019) STING polymer structure reveals mechanisms for activation, hyperactivation, and inhibition. Cell 178(2):290-301. e10
Gonugunta VK, Sakai T, Pokatayev V, Yang K, Wu J, Dobbs N, Yan N (2017) Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep 21(11):3234–3242
Pokatayev V, Yang K, Tu X, Dobbs N, Wu J, Kalb RG, Yan N (2020) Homeostatic regulation of STING protein at the resting state by stabilizer TOLLIP. Nat Immunol 21(2):158–167
Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T, Barber GN, Arai H, Taguchi T (2016) Activation of STING requires palmitoylation at the Golgi. Nat Commun 7:11932
Zhang Y, Ma Z, Wang Y, Boyer J, Ni G, Cheng L, Su S, Zhang Z, Zhu Z, Qian J, Su L, Zhang Q, Damania B, Liu P (2020) Streptavidin promotes DNA binding and activation of cGAS to enhance innate immunity. iScience 23(9):101463
Ma Z, Jacobs SR, West JA, Stopford C, Zhang Z, Davis Z, Barber GN, Glaunsinger BA, Dittmer DP, Damania B (2015) Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A 112(31):E4306-E4315
Kuznetsova AV, Meller J, Schnell PO, Nash JA, Ignacak ML, Sanchez Y, Conaway JW, Conaway RC, Czyzyk-Krzeska MF (2003) von Hippel-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci USA 100(5):2706–2711
Messaoud-Nacer Y, Culerier E, Rose S, Maillet I, Rouxel N, Briault S, Ryffel B, Quesniaux VFJ, Togbe D (2022) STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis 13(3):269
Su S, Chen J, Jiang Y, Wang Y, Vital T, Zhang J, Laggner C, Nguyen KT, Zhu Z, Prevatte AW, Barker NK, Herring LE, Davis IJ, Liu P (2021) SPOP and OTUD7A control EWS-FLI1 protein stability to govern ewing sarcoma growth. Adv Sci (Weinh) 8(14):e200846
Jiang Y, Zhang Y, Leung JY, Fan C, Popov KI, Su S, Qian J, Wang X, Holtzhausen A, Ubil E, Xiang Y, Davis I, Dokholyan NV, Wu G, Perou CM, Kim WY, Earp HS, Liu P (2019) MERTK mediated novel site Akt phosphorylation alleviates SAV1 suppression. Nat Commun 10(1):1515
Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W (2014) Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 508(7497):541–545
Brademan DR, Riley NM, Kwiecien NW, Coon JJ (2019) Interactive peptide spectral annotator: a versatile web-based tool for proteomic applications. Mol Cell Proteom 18(8 Suppl 1):S193–S201
Potjewyd F, Tuner AW, Beri J, Rectenwald, JM, Norris-Drouin JL, Cholensky SH, Margolis DM, Pearce KH, Herring LE, James LI (2020) Degradation of polycomb repressive complex 2 with an eedtargeted bivalent chemical degrader. Cell Chem Biol 27(1):47–56
Acknowledgements
We thank Liu and James lab members for critical reading of the manuscript and helpful discussions.
Funding
This work is supported by Gabrielle’s Angel Foundation Medical Research Award (PL) and University of North Carolina at Chapel Hill University Cancer Research Fund (PL). This research is based in part upon work conducted using the UNC Proteomics Core Facility, which is supported in part by NCI Center Core Support Grant (2P30CA016086-45) to the UNC Lineberger Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
Z.Z., R.L.J., L.I.J. and P.L. conceived the project and designed experiments. Z.Z., R.L.J., Z.Z. and L.E.H performed experiments. Z.Z., Z.Z., L.E.H., G.J. analyzed the data. B.D., L.I.J. and P.L. supervised this study. Z.Z., R.L.J, L.I.J, and P.L. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethical approval and consent to participate
Not applicable to this publication.
Consent for publication
All authors have approved this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Z., Johnson, R.L., Zhang, Z. et al. Development of VHL-recruiting STING PROTACs that suppress innate immunity. Cell. Mol. Life Sci. 80, 149 (2023). https://doi.org/10.1007/s00018-023-04796-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04796-7