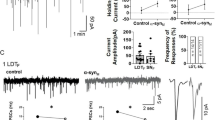
Abstract
Parkinson’s disease, Multiple System Atrophy, and Lewy Body Dementia are incurable diseases called α-synucleinopathies as they are mechanistically linked to the protein, α-synuclein (α-syn). α-syn exists in different structural forms which have been linked to clinical disease distinctions. However, sleeping disorders (SDs) are common in the prodromal phase of all three α-synucleinopathies, which suggests that sleep-controlling neurons are affected by multiple forms of α-syn. To determine whether a structure-independent neuronal impact of α-syn exists, we compared and contrasted the cellular effect of three different α-syn forms on neurotransmitter-defined cells of two sleep-controlling nuclei located in the brainstem: the laterodorsal tegmental nucleus and the pedunculopontine tegmental nucleus. We utilized size exclusion chromatography, fluorescence spectroscopy, circular dichroism spectroscopy and transmission electron microscopy to precisely characterize timepoints in the α-syn aggregation process with three different dominating forms of this protein (monomeric, oligomeric and fibril) and we conducted an in-depth investigation of the underlying neuronal mechanism behind cellular effects of the different forms of the protein using electrophysiology, multiple-cell calcium imaging, single-cell calcium imaging and live-location tracking with fluorescently-tagged α-syn. Interestingly, α-syn altered membrane currents, enhanced firing, increased intracellular calcium and facilitated cell death in a structure-independent manner in sleep-controlling nuclei, and postsynaptic actions involved a G-protein-mediated mechanism. These data are novel as the sleep-controlling nuclei are the first brain regions reported to be affected by α-syn in this structure-independent manner. These regions may represent highly important targets for future neuroprotective therapy to modify or delay disease progression in α-synucleinopathies.









Similar content being viewed by others
Abbreviations
- α-syn:
-
α-Synuclein
- LDT:
-
Laterodorsal tegmental nucleus
- PPT:
-
Pedunculopontine tegmental nucleus
- PD:
-
Parkinson’s disease
- SDs:
-
Sleeping disorders
- REM:
-
Rapid eye movement
- ThT:
-
Thioflavin T fluorescence spectroscopy
- CD:
-
Circular dichroism spectroscopy
- TEM:
-
Transmission electron microscope
- SEC:
-
Size exclusion chromatography
- GPCR:
-
G-protein coupled receptor
- bNOS:
-
Brain derived nitric oxide synthase
- sEPSCs:
-
Spontaneous excitatory post synaptic currents
- α-synF :
-
Fibril form of α-synuclein
- α-synO :
-
Oligomeric form of α-synuclein
- α-synM :
-
Monomeric form of α-synuclein
- Hz:
-
Hertz
References
Hawkes CH, Del Tredici K, Braak H (2010) A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16:79–84. https://doi.org/10.1016/j.parkreldis.2009.08.007
Mahlknecht P, Seppi K, Poewe W (2015) The concept of prodromal Parkinson’s disease. J Parkinsons Dis 5:681–697. https://doi.org/10.3233/JPD-150685
Berg D, Postuma RB, Adler CH et al (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30:1600–1611. https://doi.org/10.1002/mds.26431
Iranzo A, Santamaria J, Tolosa E (2016) Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol 15:405–419. https://doi.org/10.1016/S1474-4422(16)00057-0
Postuma RB, Gagnon J-F, Bertrand J-A et al (2015) Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 84:1104–1113. https://doi.org/10.1212/WNL.0000000000001364
dos Santos AB, Kohlmeier KA, Barreto GE (2015) Are sleep disturbances preclinical markers of Parkinson’s disease? Neurochem Res 40:421–427. https://doi.org/10.1007/s11064-014-1488-7
dos Santos AB, Barreto GE, Kohlmeier KA (2014) Treatment of sleeping disorders should be considered in clinical management of Parkinson’s disease. Front Aging Neurosci 6:273. https://doi.org/10.3389/fnagi.2014.00273
Poewe W, Seppi K, Tanner CM et al (2017) Parkinson disease. Nat Rev Dis Prim 3:17013. https://doi.org/10.1038/nrdp.2017.13
Postuma RB, Iranzo A, Hogl B et al (2015) Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol 77(5):830–839. https://doi.org/10.1002/ana.24385
Postuma RB, Iranzo A, Hu M et al (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142:744–759. https://doi.org/10.1093/brain/awz030
Fernández-Arcos A, Iranzo A, Serradell M et al (2016) The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep 39:121–132. https://doi.org/10.5665/sleep.5332
Iranzo A, Fernández-Arcos A, Tolosa E et al (2014) Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE 9(2):e89741. https://doi.org/10.1371/journal.pone.0089741
Postuma RB, Gagnon JF, Vendette M et al (2009) Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 72(15):1296–1300. https://doi.org/10.1212/01.wnl.0000340980.19702.6e
Barber TR, Lawton M, Rolinski M et al (2017) Prodromal Parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. https://doi.org/10.1093/sleep/zsx071
Schenck CH, Bundlie SR, Mahowald MW (1996) Delayed emergence of a parkinsonian disorder in 38% of 29 older, men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 46:388–393. https://doi.org/10.1212/WNL.46.2.388
Schenck CH, Boeve BF, Mahowald MW (2013) Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 14:744–748. https://doi.org/10.1016/j.sleep.2012.10.009
Abbott RD, Ross GW, White LR et al (2005) Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 65:1442–1446. https://doi.org/10.1212/01.wnl.0000183056.89590.0d
Gao J, Huang X, Park Y et al (2011) Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol 173:1032–1038. https://doi.org/10.1093/aje/kwq478
Mena-Segovia J, Bolam JP (2017) Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron 94:7–18. https://doi.org/10.1016/j.neuron.2017.02.027
Mena-Segovia J (2016) Structural and functional considerations of the cholinergic brainstem. J Neural Transm 123:731–736. https://doi.org/10.1007/s00702-016-1530-9
Armstrong DM, Saper CB, Levey AI et al (1983) Distribution of cholinergic neurons in rat brain: Demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol 216:53–68. https://doi.org/10.1002/cne.902160106
Ford B, Holmes CJ, Mainville L, Jones BE (1995) GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol 363(2):177–196. https://doi.org/10.1002/cne.903630203
Martinez-Gonzalez C, Bolam JP, Mena-Segovia J (2011) Topographical organization of the pedunculopontine nucleus. Front Neuroanat 5:543. https://doi.org/10.3389/fnana.2011.00022
Van Dort CJ, Zachs DP, Kenny JD et al (2015) Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci 112:584–589. https://doi.org/10.1073/pnas.1423136112
Zweig RM, Jankel WR, Hedreen JC et al (1989) The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol 26:41–46. https://doi.org/10.1002/ana.410260106
Braak H, Del Tredici K, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/S0197-4580(02)00065-9
Spillantini MG, Crowther RA, Jakes R et al (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A 95:6469–6473. https://doi.org/10.1073/pnas.95.11.6469
Recasens A, Dehay B (2014) Alpha-synuclein spreading in Parkinson’s disease. Front Neuroanat 8:159. https://doi.org/10.3389/fnana.2014.00159
Deschenes CL, McCurry SM (2009) Current treatments for sleep disturbances in individuals with dementia. Curr Psychiatry Rep 11:20–26. https://doi.org/10.1007/s11920-009-0004-2
Dos Santos AB, Skaanning LK, Mikkelsen E et al (2021) α-Synuclein responses in the laterodorsal tegmentum, the pedunculopontine tegmentum, and the substantia nigra: implications for early appearance of sleep disorders in Parkinson’s disease. j parkinsons dis 11:1773–1790. https://doi.org/10.3233/jpd-212554
burré j (2015) the synaptic function of α-synuclein. J Parkinsons Dis 5:699–713. https://doi.org/10.3233/JPD-150642
Guo JL, Lee VMY (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20:130–138. https://doi.org/10.1038/nm.3457
Bousset L, Pieri L, Ruiz-Arlandis G et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575. https://doi.org/10.1038/ncomms3575
Peelaerts W, Bousset L, Van Der Perren A et al (2015) α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522(7556):340–344. https://doi.org/10.1038/nature14547
Angelova PR, Ludtmann MHR, Horrocks MH et al (2016) Ca 2+ is a key factor in α-synuclein-induced neurotoxicity. J Cell Sci 129:1792–1801. https://doi.org/10.1242/jcs.180737
Deas E, Cremades N, Angelova PR et al (2016) Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxid Redox Signal 24:376–391. https://doi.org/10.1089/ars.2015.6343
Thakur P, Breger LS, Lundblad M et al (2017) Modeling Parkinson’s disease pathology by combination of fibril seeds and α-synuclein overexpression in the rat brain. Proc Natl Acad Sci 114(39):E8284–E8293. https://doi.org/10.1073/pnas.1710442114
Giehm L, Svergun DI, Otzen DE, Vestergaard B (2011) Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation. Proc Natl Acad Sci U S A 108:3246–3251. https://doi.org/10.1073/PNAS.1013225108/SUPPL_FILE/PNAS.1013225108_SI.PDF
van Maarschalkerweerd A, Vetri V, Langkilde AE et al (2014) Protein/lipid coaggregates are formed during α-synuclein-induced disruption of lipid bilayers. Biomacromol 15:3643–3654. https://doi.org/10.1021/bm500937p
Skamris T, Marasini C, Madsen KL et al (2019) Early stage alpha-synuclein amyloid fibrils are reservoirs of membrane-binding species. Sci Rep 9:1733. https://doi.org/10.1038/s41598-018-38271-2
Sepulveda FJ, Parodi J, Peoples RW et al (2010) Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS ONE 5(7):e11820. https://doi.org/10.1371/journal.pone.0011820
Pacheco CR, Morales CN, Ramírez AE et al (2015) Extracellular α-synuclein alters synaptic transmission in brain neurons by perforating the neuronal plasma membrane. J Neurochem 132(6):731–741. https://doi.org/10.1111/jnc.13060
Ipsen TH, Polli FS, Kohlmeier KA (2018) Calcium rises induced by AMPA and nicotine receptors in the ventral tegmental area show differences in mouse brain slices prenatally exposed to nicotine. Dev Neurobiol 78:828–848. https://doi.org/10.1002/dneu.22607
Veleanu M, Axen TE, Kristensen MP, Kohlmeier KA (2016) Comparison of bNOS and chat immunohistochemistry in the laterodorsal tegmentum (LDT) and the pedunculopontine tegmentum (PPT) of the mouse from brain slices prepared for electrophysiology. J Neurosci Methods 263:23–35. https://doi.org/10.1016/j.jneumeth.2016.01.020
Surguchev A, Surguchov A (2015) Effect of α-synuclein on membrane permeability and synaptic transmission: a clue to neurodegeneration? J Neurochem 132:619–621. https://doi.org/10.1111/jnc.13045
Tsigelny IF, Sharikov Y, Wrasidlo W et al (2012) Role of α-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J 279(6):1000–1013. https://doi.org/10.1111/j.1742-4658.2012.08489.x
Betzer C, Lassen LB, Olsen A et al (2018) Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep 19:e44617. https://doi.org/10.15252/embr.201744617
Pellistri F, Bucciantini M, Relini A et al (2008) Nonspecific interaction of prefibrillar amyloid aggregates with glutamatergic receptors results in Ca2+ increase in primary neuronal cells. J Biol Chem 283:29950–29960. https://doi.org/10.1074/JBC.M803992200
Leandrou E, Emmanouilidou E, Vekrellis K (2019) Voltage-gated calcium channels and α-synuclein: implications in Parkinson’s disease. Front Mol Neurosci 12:237. https://doi.org/10.3389/FNMOL.2019.00237/BIBTEX
Diogenes MJ, Dias RB, Rombo DM et al (2012) Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair ltp Via NMDA-receptor activation. J Neurosci 32:11750–11762. https://doi.org/10.1523/JNEUROSCI.0234-12.2012
Jae Kim S, Yul Kim S, Na YS et al (2006) α-Synuclein enhances dopamine D2 receptor signaling. Brain Res 1124(1):5–9. https://doi.org/10.1016/j.brainres.2006.09.079
Aman TK, Shen RY, Haj-Dahmane S (2007) D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J Pharmacol Exp Ther 320(1):376–385. https://doi.org/10.1124/jpet.106.111690
Feng LR, Federoff HJ, Vicini S, Maguire-Zeiss KA (2010) α-Synuclein mediates alterations in membrane conductance: A potential role for α-synuclein oligomers in cell vulnerability. Eur J Neurosci 32(1):10–17. https://doi.org/10.1111/j.1460-9568.2010.07266.x
Schmidt F, Levin J, Kamp F et al (2012) Single-channel electrophysiology reveals a distinct and uniform pore complex formed by α-synuclein oligomers in lipid membranes. PLoS ONE 7:e42545. https://doi.org/10.1371/journal.pone.0042545
Shrivastava AN, Redeker V, Fritz N et al (2015) α-synuclein assemblies sequester neuronal α3-Na +/K + - ATP ase and impair Na + gradient. EMBO J 34(19):2408–2423. https://doi.org/10.15252/embj.201591397
Ronzitti G, Bucci G, Stephens G, Chieregatti E (2015) Raft-partitioning of calcium channels regulates their function. Channels 9:169–170. https://doi.org/10.1080/19336950.2015.1063285
Ahn KJ, Paik SR, Chung KC, Kim J (2006) Amino acid sequence motifs and mechanistic features of the membrane translocation of α-synuclein. J Neurochem 97(1):265–279. https://doi.org/10.1111/j.1471-4159.2006.03731.x
Yin J, Han J, Zhang C et al (2011) C-terminal part of α-synuclein mediates its activity in promoting proliferation of dopaminergic cells. J Neural Transm 118(8):1155–1164. https://doi.org/10.1007/s00702-011-0592-y
Hassink GC, Raiss CC, Segers-Nolten IMJ et al (2018) Exogenous α-synuclein hinders synaptic communication in cultured cortical primary rat neurons. PLoS ONE. https://doi.org/10.1371/journal.pone.0193763
Burlet S, Tyler CJ, Leonard CS (2002) Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci 22:2862–2872. https://doi.org/10.1523/JNEUROSCI.22-07-02862.2002
Kohlmeier KA, Inoue T, Leonard CS (2004) Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and Laterodorsal Tegmentum. J Neurophysiol 92(1):221–235. https://doi.org/10.1152/jn.00076.2004
Romero-Leguizamón CR, Kohlmeier KA (2020) Stress-related endogenous neuropeptides induce neuronal excitation in the Laterodorsal Tegmentum. Eur Neuropsychopharmacol 38:86–97. https://doi.org/10.1016/J.EURONEURO.2020.07.008
Kowalski A, Betzer C, Larsen ST et al (2022) Monomeric α-synuclein activates the plasma membrane calcium pump. bioRxiv. https://doi.org/10.1101/2022.02.21.481193
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276. https://doi.org/10.1002/neu.480230915
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch Eur J Physiol 460:525–542. https://doi.org/10.1007/s00424-010-0809-1
Vergun O, Keelan J, Khodorov BI, Duchen MR (1999) Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol 519:451–466. https://doi.org/10.1111/j.1469-7793.1999.0451m.x
Vergun O, Sobolevsky AI, Yelshansky MV et al (2001) Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurones in culture. J Physiol 531:147–163. https://doi.org/10.1111/j.1469-7793.2001.0147j.x
Stout AK, Raphael HM, Kanterewicz BI et al (1998) Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. https://doi.org/10.1038/1577
Stout AK, Reynolds IJ (1999) High-affinity calcium indicators underestimate increases in intracellular calcium concentrations associated with excitotoxic glutamate stimulations. Neuroscience 1(5):366–373. https://doi.org/10.1016/S0306-4522(98)00441-2
Abramov AY, Duchen MR (2010) Impaired mitochondrial bioenergetics determines glutamate-induced delayed calcium deregulation in neurons. Biochim Biophys Acta Gen Subj 800(3):297–304. https://doi.org/10.1016/j.bbagen.2009.08.002
Büttner S, Faes L, Reichelt WN et al (2013) The Ca 2+/Mn 2+ ion-pump PMR1 links elevation of cytosolic Ca 2+ levels to -synuclein toxicity in Parkinson’s disease models. Cell Death Differ 20(3):465–477. https://doi.org/10.1038/cdd.2012.142
Volpicelli-Daley LA, Luk KC, Patel TP et al (2011) Exogenous α-synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72:57–71. https://doi.org/10.1016/j.neuron.2011.08.033
Humpel C (2015) Organotypic brain slice cultures: a review. Neuroscience 123(1):e59. https://doi.org/10.1016/j.neuroscience.2015.07.086
Kaufmann TJ, Harrison PM, Richardson MJE et al (2016) Intracellular soluble α-synuclein oligomers reduce pyramidal cell excitability. J Physiol 594:2751–2772. https://doi.org/10.1113/JP271968
Braak H, Ghebremedhin E, Rüb U et al (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134. https://doi.org/10.1007/s00441-004-0956-9
Mollenhauer B, Cullen V, Kahn I et al (2008) Direct quantification of CSF α-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 213:315–325. https://doi.org/10.1016/j.expneurol.2008.06.004
Wang Y, Shi M, Chung KA et al (2012) Phosphorylated -Synuclein in Parkinson’s Disease. Sci Transl Med 4:121ra20. https://doi.org/10.1126/scitranslmed.3002566
Fanning S, Selkoe D, Dettmer U (2020) Parkinson’s disease: proteinopathy or lipidopathy? npj Parkinsons Dis 61(6):1–9. https://doi.org/10.1038/s41531-019-0103-7
Von Euler CM, Söderberg L, Fälting J et al (2020) Alpha-synuclein protofibrils in cerebrospinal fluid: a potential biomarker for Parkinson’s disease. J Parkinsons Dis 10:1429–1442. https://doi.org/10.3233/JPD-202141
Schenck CH (2016) Expanded insights into idiopathic REM sleep behavior disorder. Sleep 39:7–9. https://doi.org/10.5665/sleep.5300
Karachi C, Grabli D, Bernard FA et al (2010) Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. https://doi.org/10.1172/JCI42642
Gilman S, Koeppe RA, Nan B et al (2010) Cerebral cortical and subcortical cholinergic deficits in Parkinsonian syndromes. Neurology 74:1416–1423. https://doi.org/10.1212/WNL.0b013e3181dc1a55
Müller MLTM, Bohnen NI (2013) Cholinergic dysfunction in parkinson’s disease. Curr Neurol Neurosci Rep 13(9):377. https://doi.org/10.1007/s11910-013-0377-9
Postuma RB, Aarsland D, Barone P et al (2012) Identifying prodromal Parkinson’s disease: Pre-Motor disorders in Parkinson’s disease. Mov Disord 27:617–626. https://doi.org/10.1002/mds.24996
Berkhoudt Lassen L, Gregersen E, Kathrine Isager A et al (2018) ELISA method to detect α-synuclein oligomers in cell and animal models. PLoS ONE 13:e0196056. https://doi.org/10.1371/JOURNAL.PONE.0196056
Emadi S, Kasturirangan S, Wang MS et al (2009) Detecting morphologically distinct oligomeric forms of alpha-synuclein. J Biol Chem 284:11048–11058. https://doi.org/10.1074/JBC.M806559200
Khatri A, Punjabi N, Ghosh D et al (2018) Detection and differentiation of α-Synuclein monomer and fibril by chitosan film coated nanogold array on optical sensor platform. Sensors Actuators B Chem 255:692–700. https://doi.org/10.1016/J.SNB.2017.08.051
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil for funding support in the form of a Ph.D Grant to Altair Brito dos Santos. LKS, TS and AEL acknowledge funding from the Lundbeck Foundation Initiative BRAINSTRUC (2015-2666).
Author information
Authors and Affiliations
Contributions
ABDS, MPK and KAK initiated the study and designed the experimental strategy. LKS, TSP, and AEL expressed and characterized protein structure. ABDS, ST and EM performed and analyzed electrophysiological, and calcium imaging experiments. ABDS and CRRL performed neurodegeneration imaging experiments and data analyzes. ABDS and KAK performed statistical evaluations. LKS, AEL, EM, ST, MPK, ABDS and KAK prepared figures. ABDS, AEL, MPK and KAK wrote the paper, and all authors provided critical feedback on early drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest in regard to this manuscript.
data availability
upon acceptance, data are available at a repository at the university of copenhagen and made available upon reasonable request to the authors.
ethics approval
Animals were used under approval in accordance with European Communities Council Directive (86/609/EEC).
Statement about data used in another study
In the data set reporting amplitudes of effects with monomer and fibril, some of the same data were used in a previously published study (2021;11(4):1773–1790. https://doi.org/10.3233/JPD-212554). However, the present report represents a distinctly different study as it examines a hypothesis, and presents conclusions which are different from those in the earlier publication. In addition, we present the results of many new experiments in this report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dos Santos, A.B., Skaanning, L.K., Thaneshwaran, S. et al. Sleep-controlling neurons are sensitive and vulnerable to multiple forms of α-synuclein: implications for the early appearance of sleeping disorders in α-synucleinopathies. Cell. Mol. Life Sci. 79, 450 (2022). https://doi.org/10.1007/s00018-022-04467-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04467-z