Abstract
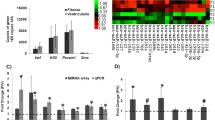
Thoracic aortic aneurysms (TAA) in Marfan syndrome, caused by fibrillin-1 mutations, are characterized by elevated cytokines and fragmentated elastic laminae in the aortic wall. This study explored whether and how specific fibrillin-1-regulated miRNAs mediate inflammatory cytokine expression and elastic laminae degradation in TAA. miRNA expression profiling at early and late TAA stages using a severe Marfan mouse model (Fbn1mgR/mgR) revealed a spectrum of differentially regulated miRNAs. Bioinformatic analyses predicted the involvement of these miRNAs in inflammatory and extracellular matrix-related pathways. We demonstrate that upregulation of pro-inflammatory cytokines and matrix metalloproteinases is a common characteristic of mouse and human TAA tissues. miR-122, the most downregulated miRNA in the aortae of 10-week-old Fbn1mgR/mgR mice, post-transcriptionally upregulated CCL2, IL-1β and MMP12. Similar data were obtained at 70 weeks of age using Fbn1C1041G/+ mice. Deficient fibrillin-1–smooth muscle cell interaction suppressed miR-122 levels. The marker for tissue hypoxia HIF-1α was upregulated in the aortic wall of Fbn1mgR/mgR mice, and miR-122 was reduced under hypoxic conditions in cell and organ cultures. Reduced miR-122 was partially rescued by HIF-1α inhibitors, digoxin and 2-methoxyestradiol in aortic smooth muscle cells. Digoxin-treated Fbn1mgR/mgR mice demonstrated elevated miR-122 and suppressed CCL2 and MMP12 levels in the ascending aortae, with reduced elastin fragmentation and aortic dilation. In summary, this study demonstrates that miR-122 in the aortic wall inhibits inflammatory responses and matrix remodeling, which is suppressed by deficient fibrillin-1–cell interaction and hypoxia in TAA.








Similar content being viewed by others
Data availability
The microarray data have been deposited in the NCBI's Gene Expression Omnibus database https://www.ncbi.nlm.nih.gov/geo and are accessible through GEO Series accession number GSE199285. All other data sets of this study will be made available upon request to the corresponding author.
Abbreviations
- AAA:
-
Abdominal aortic aneurysm
- DMSO:
-
Dimethyl sulfoxide
- ECM:
-
Extracellular matrix
- FAK:
-
Focal adhesion kinase
- FBS:
-
Fetal bovine serum
- GSEA:
-
Gene set enrichment analyses
- HIF-1α:
-
Hypoxia-inducible factor 1α
- MFS:
-
Marfan syndrome
- miRNA:
-
MicroRNA
- MMP:
-
Matrix metalloproteinase
- PBS:
-
Phosphate-buffered saline
- PSG:
-
Penicillin–streptomycin–glutamine
- TAA:
-
Thoracic aortic aneurysm
- TGF-β:
-
Transforming growth factor β
- αSMA:
-
α-Smooth muscle actin
References
Milewicz DM, Ramirez F (2019) Therapies for thoracic aortic aneurysms and acute aortic dissections. Arterioscler Thromb Vasc Biol 39(2):126–136. https://doi.org/10.1161/ATVBAHA.118.310956
Pinard A, Jones GT, Milewicz DM (2019) Genetics of thoracic and abdominal aortic diseases. Circ Res 124(4):588–606
Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA (1991) Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352:337–339. https://doi.org/10.1038/352337a0
Milewicz DM, Braverman AC, De Backer J, Morris SA, Boileau C, Maumenee IH, Jondeau G, Evangelista A, Pyeritz RE, European Reference Network for Rare Multisystemic Vascular Disease HRDWG (2021) Marfan syndrome. Nat Rev Dis Primers 7(1):64. https://doi.org/10.1038/s41572-021-00298-7
Collod-Beroud G, Le Bourdelles S, Ades L, Ala-Kokko L, Booms P, Boxer M, Child A, Comeglio P, De Paepe A, Hyland JC, Holman K, Kaitila I, Loeys B, Matyas G, Nuytinck L, Peltonen L, Rantamaki T, Robinson P, Steinmann B, Junien C, Beroud C, Boileau C (2003) Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat 22(3):199–208. https://doi.org/10.1002/humu.10249
Pyeritz R, Dietz H (2002) Marfan syndrome and other microfibrillar disorders. In: Royce PM, Steinmann B (eds) Connective tissue and its heritable disorders, vol 2. Wiley-Liss Inc, New York, pp 585–626
Davis EC (1993) Smooth muscle cell to elastic lamina connections in developing mouse aorta. Lab Invest 68:89–99
Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, Kamm K, Stull JT (2017) Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler Thromb Vasc Biol 37(1):26–34. https://doi.org/10.1161/ATVBAHA.116.303229
Khau Van Kien P, Mathieu F, Zhu L, Lalande A, Betard C, Lathrop M, Brunotte F, Wolf JE, Jeunemaitre X (2005) Mapping of familial thoracic aortic aneurysm/dissection with patent ductus arteriosus to 16p12.2-p13.13. Circulation 112(2):200–206
Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM (2007) Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 39(12):1488–1493
Li Y, Gao S, Han Y, Song L, Kong Y, Jiao Y, Huang S, Du J, Li Y (2021) Variants of focal adhesion scaffold genes cause thoracic aortic aneurysm. Circ Res 128(1):8–23. https://doi.org/10.1161/CIRCRESAHA.120.317361
Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC (2003) Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33(3):407–411. https://doi.org/10.1038/ng1116
Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC (2011) Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332(6027):358–361. https://doi.org/10.1126/science.1192149
He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, Geng YJ, Milewicz DM (2006) Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg 131(3):671–678
Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F (1999) Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA 96(7):3819–3823
Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC (2004) Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114(2):172–181
Ju X, Ijaz T, Sun H, Lejeune W, Vargas G, Shilagard T, Recinos AR, Milewicz DM, Brasier AR, Tilton RG (2014) IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome. J Am Heart Assoc. https://doi.org/10.1161/JAHA.113.000476
Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, Lu G, Owens GK, Upchurch GR Jr, Ailawadi G (2014) Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130(11 Suppl 1):S51–S59. https://doi.org/10.1161/CIRCULATIONAHA.113.006800
Alexander MR, Murgai M, Moehle CW, Owens GK (2012) Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-kappaB-dependent mechanisms. Physiol Genomics 44(7):417–429. https://doi.org/10.1152/physiolgenomics.00160.2011
Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T (2003) Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation 108(Suppl 1):II329–II334
Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT (2012) MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res 110(12):e92–e101
Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC (2001) Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res 88(1):37–43
Jimenez-Altayo F, Meirelles T, Crosas-Molist E, Sorolla MA, Del Blanco DG, Lopez-Luque J, Mas-Stachurska A, Siegert AM, Bonorino F, Barbera L, Garcia C, Condom E, Sitges M, Rodriguez-Pascual F, Laurindo F, Schroder K, Ros J, Fabregat I, Egea G (2018) Redox stress in Marfan syndrome: dissecting the role of the NADPH oxidase NOX4 in aortic aneurysm. Free Radic Biol Med 118:44–58. https://doi.org/10.1016/j.freeradbiomed.2018.02.023
Kaelin WG Jr (2005) ROS: really involved in oxygen sensing. Cell Metab 1(6):357–358
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148(3):399–408. https://doi.org/10.1016/j.cell.2012.01.021
Liu K, Fang C, Shen Y, Liu Z, Zhang M, Ma B, Pang X (2017) Hypoxia-inducible factor 1a induces phenotype switch of human aortic vascular smooth muscle cell through PI3K/AKT/AEG-1 signaling. Oncotarget 8(20):33343–33352. https://doi.org/10.18632/oncotarget.16448
Wang W, Xu B, Xuan H, Ge Y, Wang Y, Wang L, Huang J, Fu W, Michie SA, Dalman RL (2018) Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg 68(5):1538-1550 e1532. https://doi.org/10.1016/j.jvs.2017.09.030
Tsai SH, Huang PH, Hsu YJ, Peng YJ, Lee CH, Wang JC, Chen JW, Lin SJ (2016) Inhibition of hypoxia inducible factor-1alpha attenuates abdominal aortic aneurysm progression through the down-regulation of matrix metalloproteinases. Sci Rep 6:28612. https://doi.org/10.1038/srep28612
Li J, Kim SG, Blenis J (2014) Rapamycin: one drug, many effects. Cell Metab 19(3):373–379. https://doi.org/10.1016/j.cmet.2014.01.001
Liu M, Li L, Zhu J, He C, Xu Q, Sun A, Kong W, Li W, Zhang X (2019) Rapamycin attenuates a murine model of thoracic aortic aneurysm by downregulating the miR-126-3p mediated activation of MAPK/ERK signalling pathway. Biochem Biophys Res Commun 512(3):498–504. https://doi.org/10.1016/j.bbrc.2019.03.083
Lawrence DM, Singh RS, Franklin DP, Carey DJ, Elmore JR (2004) Rapamycin suppresses experimental aortic aneurysm growth. J Vasc Surg 40(2):334–338. https://doi.org/10.1016/j.jvs.2004.05.020
Imanishi M, Chiba Y, Tomita N, Matsunaga S, Nakagawa T, Ueno M, Yamamoto K, Tamaki T, Tomita S (2016) Hypoxia-inducible factor-1 alpha in smooth muscle cells protects against aortic aneurysms. Arterioscler Thromb Vasc Biol 36(11):2158–2162
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, Spin JM, Alvira CM, Robbins RC, Fischbein MP (2012) miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res 110(2):312–324. https://doi.org/10.1161/circresaha.111.253740
Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS (2012) Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 122(2):497–506
Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell MV, Dalman RL, Spin JM, Tsao PS (2012) MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med 4(122):122ra122. https://doi.org/10.1126/scitranslmed.3003441
Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL, Tsao PS (2014) miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 5:5214. https://doi.org/10.1038/ncomms6214
Zhang RM, Zeyer KA, Odenthal N, Zhang Y, Reinhardt DP (2021) The fibrillin-1 RGD motif posttranscriptionally regulates ERK1/2 signaling and fibroblast proliferation via miR-1208. FASEB J 35(5):e21598. https://doi.org/10.1096/fj.202100282R
Zeyer KA, Zhang RM, Kumra H, Hassan A, Reinhardt DP (2019) The fibrillin-1 RGD integrin binding site regulates gene expression and cell function through microRNAs. J Mol Biol 431(2):401–421. https://doi.org/10.1016/j.jmb.2018.11.021
Eichhorn SW, Guo H, McGeary SE, Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villen J, Bartel DP (2014) mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol Cell 56(1):104–115. https://doi.org/10.1016/j.molcel.2014.08.028
Karagkouni D, Paraskevopoulou MD, Chatzopoulos S, Vlachos IS, Tastsoglou S, Kanellos I, Papadimitriou D, Kavakiotis I, Maniou S, Skoufos G, Vergoulis T, Dalamagas T, Hatzigeorgiou AG (2018) DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res 46(D1):D239–D245. https://doi.org/10.1093/nar/gkx1141
Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM (2008) Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood 112(8):3455–3464. https://doi.org/10.1182/blood-2007-12-129080
Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ (2003) Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA 100(23):13298–13302
Oller J, Gabande-Rodriguez E, Ruiz-Rodriguez MJ, Desdin-Mico G, Aranda JF, Rodrigues-Diez R, Ballesteros-Martinez C, Blanco EM, Roldan-Montero R, Acuna P, Forteza Gil A, Martin-Lopez CE, Nistal JF, Lino Cardenas CL, Lindsay ME, Martin-Ventura JL, Briones AM, Redondo JM, Mittelbrunn M (2021) Extracellular tuning of mitochondrial respiration leads to aortic aneurysm. Circulation 143(21):2091–2109. https://doi.org/10.1161/CIRCULATIONAHA.120.051171
Chance B (1965) Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol 49(1 Suppl):163–195
Goda N, Kanai M (2012) Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol 95(5):457–463. https://doi.org/10.1007/s12185-012-1069-y
Kroner BL, Tolunay HE, Basson CT, Pyeritz RE, Holmes KW, Maslen CL, Milewicz DM, LeMaire SA, Hendershot T, Desvigne-Nickens P, Devereux RB, Dietz HC, Song HK, Ringer D, Mitchell M, Weinsaft JW, Ravekes W, Menashe V, Eagle KA (2011) The National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC): results from phase I and scientific opportunities in phase II. Am Heart J 162(4):627-632 e621. https://doi.org/10.1016/j.ahj.2011.07.002
Cook JR, Clayton NP, Carta L, Galatioto J, Chiu E, Smaldone S, Nelson CA, Cheng SH, Wentworth BM, Ramirez F (2015) Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb Vasc Biol 35(4):911–917. https://doi.org/10.1161/ATVBAHA.114.305150
Nathan C, Sporn M (1991) Cytokines in context. J Cell Biol 113(5):981–986. https://doi.org/10.1083/jcb.113.5.981
Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT (2008) Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg 47(1):166–172
Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, Spinale FG, Gorman RC (2006) Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation 114:I365–I370. https://doi.org/10.1161/circulationaha.105.000810
Proietta M, Tritapepe L, Cifani N, Ferri L, Taurino M, Del Porto F (2014) MMP-12 as a new marker of Stanford-A acute aortic dissection. Ann Med 46(1):44–48. https://doi.org/10.3109/07853890.2013.876728
Wu L, Tanimoto A, Murata Y, Sasaguri T, Fan J, Sasaguri Y, Watanabe T (2003) Matrix metalloproteinase-12 gene expression in human vascular smooth muscle cells. Genes Cells 8(3):225–234
Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW (1998) Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest 102(11):1900–1910
Wen J, Friedman JR (2012) miR-122 regulates hepatic lipid metabolism and tumor suppression. J Clin Invest 122(8):2773–2776. https://doi.org/10.1172/JCI63966
Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP (2012) MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 122(8):2884–2897. https://doi.org/10.1172/JCI63455
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15–20
Yang CQ, Li W, Li SQ, Li J, Li YW, Kong SX, Liu RM, Wang SM, Lv WM (2014) MCP-1 stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol Biochem 34(2):266–276. https://doi.org/10.1159/000362997
Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, Amento E, Libby P (1994) Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res 75(1):181–189
Pfaff M, Reinhardt DP, Sakai LY, Timpl R (1996) Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett 384:247–250
Sakamoto H, Broekelmann T, Cheresh DA, Ramirez F, Rosenbloom J, Mecham RP (1996) Cell-type specific recognition of RGD- and non-RGD-containing cell binding domains in fibrillin-1. J Biol Chem 271:4916–4922
Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ (2007) High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer 6:5. https://doi.org/10.1186/1476-4598-6-5
Liang W, Guo J, Li J, Bai C, Dong Y (2016) Downregulation of miR-122 attenuates hypoxia/reoxygenation (H/R)-induced myocardial cell apoptosis by upregulating GATA-4. Biochem Biophys Res Commun 478(3):1416–1422. https://doi.org/10.1016/j.bbrc.2016.08.139
Wei Z, Wang Y, Zhang K, Liao Y, Ye P, Wu J, Wang Y, Li F, Yao Y, Zhou Y, Liu J (2014) Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 34(11):2429–2438. https://doi.org/10.1161/atvbaha.114.304435
Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, Powell JT, Yoshimura K, Hultgren R (2018) Abdominal aortic aneurysms. Nat Rev Dis Primers 4(1):34. https://doi.org/10.1038/s41572-018-0030-7
Cikach FS, Koch CD, Mead TJ, Galatioto J, Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F, Roselli EE, Apte SS (2018) Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. https://doi.org/10.1172/jci.insight.97167
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(43):15545–15550
Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG (2015) DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43(W1):W460–W466. https://doi.org/10.1093/nar/gkv403
Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C, Dalamagas T, Hatzigeorgiou AG (2013) DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res 41(Web Server issue):W169–W173. https://doi.org/10.1093/nar/gkt393
Zhang RM, Kumra H, Reinhardt DP (2019) Quantification of extracellular matrix fiber systems related to ADAMTS proteins. Methods Mol Biol 2043:237–250. https://doi.org/10.1007/978-1-4939-9698-8_19
Shi Y, Jones W, Beatty W, Tan Q, Mecham RP, Kumra H, Reinhardt DP, Gibson MA, Reilly MA, Rodriguez J, Bassnett S (2021) Latent-transforming growth factor beta-binding protein-2 (LTBP-2) is required for longevity but not for development of zonular fibers. Matrix Biol 95:15–31. https://doi.org/10.1016/j.matbio.2020.10.002
Kumra H, Nelea V, Hakami H, Pagliuzza A, Djokic J, Xu J, Yanagisawa H, Reinhardt DP (2019) Fibulin-4 exerts a dual role in LTBP-4L-mediated matrix assembly and function. Proc Natl Acad Sci USA 116(41):20428–20437. https://doi.org/10.1073/pnas.1901048116
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Dagher M, Kleinman M, Ng A, Juncker D (2018) Ensemble multicolour FRET model enables barcoding at extreme FRET levels. Nat Nanotechnol 13(10):925–932. https://doi.org/10.1038/s41565-018-0205-0
Acknowledgements
We thank Dr. Francesco Ramirez in the Department of Pharmacological Sciences, Icahn School of Medicine at Mt Sinai for generously providing Fbn1mgR/+ mice. We are grateful to Dr. Jean-Martin Laberge at the Montreal Children’s Hospital for enabling fibroblast collections from donors. We also thank Dr. Anie Philip for providing primary fibroblast controls, and Dr. Ling Li for help with tissue preparations.
Funding
This work was supported by the Canadian Institutes of Health Research (PJT-162099), the Marfan Foundation (USA), The Natural Sciences and Engineering Research Council of Canada (RGPIN-06278), the Genetic Aortic Disorders Association Canada, and the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Study conception and design: RMZ, DPR. Acquisition of data: RMZ, KT, BR, MLM, SK. Analysis and interpretation of data: RMZ, DPR. Manuscript writing: RMZ, DPR. Manuscript editing: KT, BR, SK, MLM, NEHD.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, RM., Tiedemann, K., Muthu, M.L. et al. Fibrillin-1-regulated miR-122 has a critical role in thoracic aortic aneurysm formation. Cell. Mol. Life Sci. 79, 314 (2022). https://doi.org/10.1007/s00018-022-04337-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04337-8