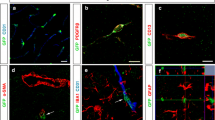
Abstract
Pericytes are multipotent perivascular cells that play important roles in CNS injury. However, controversial findings exist on how pericytes change and whether they differentiated into microglia-like cells after ischemic stroke. This discrepancy is mainly due to the lack of pericyte-specific markers: the “pericyte” population identified in previous studies contained vascular smooth muscle cells (vSMCs) and/or fibroblasts. Therefore, it remains unclear which cell type differentiates into microglia-like cells after stroke. In this study, lineage-tracing technique was used to mark α-smooth muscle actin (SMA)low/undetectable pericytes, vSMCs, and fibroblasts, and their fates were analyzed after ischemic stroke. We found that SMAlow/undetectable pericytes and fibroblasts but not vSMCs substantially proliferated at the subacute phase after injury, and that SMAlow/undetectable pericyte but not vSMCs or fibroblasts differentiated into Iba1+ cells after ischemic stroke. Further imaging flow cytometry analysis revealed that SMAlow/undetectable pericytes differentiated into both microglia and macrophages at day 7 after stroke. These results demonstrate that SMAlow/undetectable pericytes rather than vSMCs or fibroblasts differentiate into both microglia-like and macrophage-like cells after stroke, suggesting that these pericytes may be targeted in the treatment of ischemic stroke.






Similar content being viewed by others
Data availability
All data generated in this study are available in this article and the online supplementary material.
Abbreviations
- Cas3:
-
Caspase 3
- CCA:
-
Common carotid artery
- Col1α1:
-
Alpha-1 type I collagen
- DPI:
-
Days post-injury
- ECA:
-
External carotid artery
- ECM:
-
Extracellular matrix
- ICA:
-
Internal carotid artery
- MCAO:
-
Middle cerebral artery occlusion
- SMA:
-
α-Smooth muscle actin
- vSMCs:
-
Vascular smooth muscle cell
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S (2014) Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292. https://doi.org/10.1161/01.cir.0000441139.02102.80
Cronin CA, Sheth KN, Zhao X, Messé SR, Olson DM, Hernandez AF, Bhatt DL, Schwamm LH, Smith EE (2014) Adherence to third European Cooperative Acute Stroke Study 3-to 4.5-hour exclusions and association with outcome: data from Get with the Guidelines-Stroke. Stroke 45(9):2745–2749. https://doi.org/10.1161/STROKEAHA.114.005443
Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL (2014) Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res 51(3):163–174. https://doi.org/10.1159/000362276
Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19(6):771–783. https://doi.org/10.1038/nn.4288
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood–brain barrier. Nature 468(7323):557–561. https://doi.org/10.1038/nature09522
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68(3):409–427. https://doi.org/10.1016/j.neuron.2010.09.043
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468(7323):562–566. https://doi.org/10.1038/nature09513
Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443(7112):700–704. https://doi.org/10.1038/nature05193
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’farrell FM, Buchan AM, Lauritzen M, Attwell D, (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508(7494):55–60. https://doi.org/10.1038/nature13165
Girouard H, Iadecola C (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100(1):328–335. https://doi.org/10.1152/japplphysiol.00966.2005
Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, Kawahara M, Taguchi A, Matsuyama T (2015) Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 33(6):1962–1974. https://doi.org/10.1002/stem.1977
Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, Kuwahara-Otani S, Hayakawa T, Yagi H, Matsuyama T, Nakagomi T (2016) Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflamm 13(57):1–13. https://doi.org/10.1186/s12974-016-0523-9
Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T (2016) What is a pericyte? J Cereb Blood Flow Metab 36(2):451–455. https://doi.org/10.1177/0271678X15610340
Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM (2017) Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20(3):345–359. https://doi.org/10.1016/j.stem.2016.12.006
Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, Paul G (2014) Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol 128(3):381–396. https://doi.org/10.1007/s00401-014-1295-x
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21(2):193–215. https://doi.org/10.1016/j.devcel.2011.07.001
Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Lavina B, Gouveia L, Sun Y, Raschperger E, Rasanen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C (2018) A molecular atlas of cell types and zonation in the brain vasculature. Nature 554(7693):475–480. https://doi.org/10.1038/nature25739
Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J (2015) Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87(1):95–110. https://doi.org/10.1016/j.neuron.2015.06.001
Xu L, Yao Y (2021) Central nervous system fibroblast-like cells in stroke and other neurological disorders. Stroke 57(7):2456–2464. https://doi.org/10.1161/STROKEAHA.120.033431
Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O (2007) Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38(11):3032–3039. https://doi.org/10.1161/STROKEAHA.107.488445
Morris GP, Wright AL, Tan RP, Gladbach A, Ittner LM, Vissel B (2016) A Comparative study of variables influencing ischemic injury in the longa and koizumi methods of intraluminal filament middle cerebral artery occlusion in mice. PLoS One 11(2):1–34. https://doi.org/10.1371/journal.pone.0148503
McBride DW, Klebe D, Tang J, Zhang JH (2015) Correcting for brain swelling’s effects on infarct volume calculation after middle cerebral artery occlusion in rats. Transl Stroke Res 6(4):323–338. https://doi.org/10.1007/s12975-015-0400-3
Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C (1998) A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke 29(7):1454–1460. https://doi.org/10.1161/01.STR.29.7.1454
Rousselet E, Kriz J, Seidah NG (2012) Mouse model of intraluminal MCAO: cerebral infarct evaluation by cresyl violet staining. J Vis Exp 6(69):e4038. https://doi.org/10.3791/4038
Barreto GE, Sun X, Xu L, Giffard RG (2011) Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS One 6(11):e27881. https://doi.org/10.1371/journal.pone.0027881
Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS (2006) Aminoguanidine inhibits caspase-3 and calpain activation without affecting microglial activation following neonatal transient cerebral ischemia. J Neurochem 96(5):1467–1479. https://doi.org/10.1111/j.1471-4159.2006.03672.x
Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y, Nabekura J, Sato K, Okajima F, Takebayashi H, Okano H, Koizumi S (2017) Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat Commun 8:28. https://doi.org/10.1038/s41467-017-00037-1
Grabert K, McColl BW (2018) Isolation and phenotyping of adult mouse microglial cells. In: Rousselet G (ed) Macrophages. Methods in molecular biology, vol 1784. Humana Press, New York
Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S (2008) G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14(1):64–68. https://doi.org/10.1038/nm1666
Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M (2002) Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 99(10):7142–7147. https://doi.org/10.1073/pnas.102650499
Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA 113(12):E1738-1746. https://doi.org/10.1073/pnas.1525528113
Kaiser T, Feng G (2019) Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6(4):ENEURO.0448-18.2019. https://doi.org/10.1523/ENEURO.0448-18.2019
Fernandez-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, Engel O, Stenzel W, Genove G, Priller J (2013) Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 33(3):428–439. https://doi.org/10.1038/jcbfm.2012.187
Özen I, Roth M, Barbariga M, Gaceb A, Deierborg T, Genové G, Paul G (2018) Loss of regulator of G-protein signaling 5 leads to neurovascular protection in stroke. Stroke 49(9):2182–2190. https://doi.org/10.1161/STROKEAHA.118.020124
Muramatsu R, Yamashita T (2014) Pericyte function in the physiological central nervous system. Neurosci Res 81:38–41. https://doi.org/10.1016/j.neures.2014.01.007
Gautam J, Yao Y (2018) Roles of pericytes in stroke pathogenesis. Cell Transplant 27(12):1798–1808. https://doi.org/10.1177/0963689718768455
Kelly KK, MacPherson AM, Grewal H, Strnad F, Jones JW, Yu J, Pierzchalski K, Kane MA, Herson PS, Siegenthaler JA (2016) Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neurosci 17:49. https://doi.org/10.1186/s12868-016-0284-5
Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P (2013) Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 33(34):13882–13887
Dorrier CE, Aran D, Haenelt EA, Sheehy RN, Hoi KK, Pintarić L, Chen Y, Lizama CO, Cautivo KM, Weiner GA (2021) CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat Neurosci 24(2):234–244. https://doi.org/10.1038/s41593-020-00770-9
Aronowski J, Strong R, Grotta JC (1997) Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab 17(10):1048–1056. https://doi.org/10.1097/00004647-199710000-00006
Hartmann DA, Berthiaume A-A, Grant RI, Harrill SA, Koski T, Tieu T, McDowell KP, Faino AV, Kelly AL, Shih AY (2021) Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat Neurosci 24(5):633–645. https://doi.org/10.1038/s41593-020-00793-2
Grant RI, Hartmann DA, Underly RG, Berthiaume A-A, Bhat NR, Shih AY (2019) Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab 39(3):411–425. https://doi.org/10.1177/0271678X17732229
Gonzales AL, Klug NR, Moshkforoush A, Lee JC, Lee FK, Shui B, Tsoukias NM, Kotlikoff MI, Hill-Eubanks D, Nelson MT (2020) Contractile pericytes determine the direction of blood flow at capillary junctions. Proc Natl Acad Sci USA 117(43):27022–27033. https://doi.org/10.1073/pnas.1922755117
Cho S, Park E-M, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C (2005) The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci 25(10):2504–2512. https://doi.org/10.1523/JNEUROSCI.0035-05.2005
Cho S, Park E-M, Zhou P, Frys K, Ross ME, Iadecola C (2005) Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab 25(4):493–501. https://doi.org/10.1038/sj.jcbfm.9600058
Kunz A, Abe T, Hochrainer K, Shimamura M, Anrather J, Racchumi G, Zhou P, Iadecola C (2008) Nuclear factor-κB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci 28(7):1649–1658. https://doi.org/10.1523/JNEUROSCI.5205-07.2008
Kunz A, Anrather J, Zhou P, Orio M, Iadecola C (2007) Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab 27(3):545–551. https://doi.org/10.1038/sj.jcbfm.9600369
Park E-M, Cho S, Frys K, Racchumi G, Zhou P, Anrather J, Iadecola C (2004) Interaction between inducible nitric oxide synthase and poly (ADP-ribose) polymerase in focal ischemic brain injury. Stroke 35(12):2896–2901. https://doi.org/10.1161/01.STR.0000147042.53659.6c
Park E-M, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C (2006) Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab 26(3):392–401. https://doi.org/10.1038/sj.jcbfm.9600194
Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, Iadecola C (2010) Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke 41(5):898–904. https://doi.org/10.1161/STROKEAHA.109.572552
McColl BW, Carswell HV, McCulloch J, Horsburgh K (2004) Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res 997(1):15–23. https://doi.org/10.1016/j.brainres.2003.10.028
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87(1):179–197. https://doi.org/10.1016/j.pbb.2007.04.015
Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T (1998) Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab 18(5):570–579. https://doi.org/10.1097/00004647-199805000-00012
Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann K-A (1998) A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab 18(4):367–375. https://doi.org/10.1097/00004647-199804000-00004
Zhang F, Guo R-M, Yang M, Wen X-H, Shen J (2012) A stable focal cerebral ischemia injury model in adult mice: assessment using 7T MR imaging. Am J Neuroradiol 33(5):935–939. https://doi.org/10.3174/ajnr.A2887
Acknowledgements
We would like to thank Dr. Volkhard Lindner for the PDGFRβ-Cre mice.
Funding
This work was partially supported by NIH Grants (R01HL146574, RF1AG065345, R21AG064422, and R21AG073862) and AHA Grant (16SDG29320001) to YY.
Author information
Authors and Affiliations
Contributions
Conceptualization, YY; Methodology, AN and YY; Analysis, AN; Writing, AN and YY; Funding Acquisition, YY; Supervision, YY.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures were approved by the Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nirwane, A., Yao, Y. SMAlow/undetectable pericytes differentiate into microglia- and macrophage-like cells in ischemic brain. Cell. Mol. Life Sci. 79, 264 (2022). https://doi.org/10.1007/s00018-022-04322-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04322-1