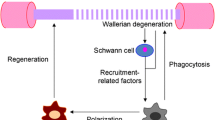
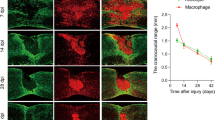
Abstract
Accumulating evidences suggest that M2 macrophages are involved with repair processes in the nervous system. However, whether M2 macrophages can promote axon regeneration by directly stimulating axons nor its precise molecular mechanism remains elusive. Here, the current study demonstrated that typical M2 macrophages, which were generated by IL4 simulation, had the capacity to stimulate axonal growth by their direct effect on axons and that the graft of IL4 stimulated macrophages into the region of Wallerian degeneration enhanced axon regeneration and improved functional recovery after PNI. Importantly, uPA (urokinase plasminogen activator)-uPA receptor (uPAR) was identified as the central axis underlying the axon regeneration effect of IL4 stimulated macrophages. IL4 stimulated macrophages secreted uPA, and its inhibition abolished their axon regeneration effect. Injured but not intact axons expressed uPAR to be sensitive to uPA. These results unveil a cellular and molecular mechanism underlying the macrophage related axon regeneration and provide a basis of a novel therapy for PNI.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Campana L, Esser H, Huch M, Forbes S (2021) Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. https://doi.org/10.1038/s41580-021-00373-7
Zhang S, Bories G, Lantz C, Emmons R, Becker A, Liu E et al (2019) Immunometabolism of phagocytes and relationships to cardiac repair. Front Cardiovasc Med 6:42. https://doi.org/10.3389/fcvm.2019.00042
Parimon T, Hohmann MS, Yao C (2021) Cellular senescence: pathogenic mechanisms in lung fibrosis. Int J Mol Sci 22(12):6214. https://doi.org/10.3390/ijms22126214
Zigmond RE, Echevarria FD (2019) Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol 173:102–121. https://doi.org/10.1016/j.pneurobio.2018.12.001
Mesquida-Veny F, Del Rio JA, Hervera A (2021) Macrophagic and microglial complexity after neuronal injury. Prog Neurobiol 200:101970. https://doi.org/10.1016/j.pneurobio.2020.101970
Perrin FE, Lacroix S, Aviles-Trigueros M, David S (2005) Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 128(Pt 4):854–866. https://doi.org/10.1093/brain/awh407
Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE (2013) A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci 33(41):16236–16248. https://doi.org/10.1523/JNEUROSCI.3319-12.2013
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6(13):787–795. https://doi.org/10.12703/P6-13
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795. https://doi.org/10.1172/JCI59643
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41(1):14–20. https://doi.org/10.1016/j.immuni.2014.06.008
Ivashkiv LB (2013) Epigenetic regulation of macrophage polarization and function. Trends Immunol 34(5):216–223. https://doi.org/10.1016/j.it.2012.11.001
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F (2018) The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9:419. https://doi.org/10.3389/fphys.2018.00419
Ydens E, Amann L, Asselbergh B, Scott CL, Martens L, Sichien D et al (2020) Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat Neurosci 23(5):676–689. https://doi.org/10.1038/s41593-020-0618-6
Gay D, Ghinatti G, Guerrero-Juarez CF, Ferrer RA, Ferri F, Lim CH et al (2020) Phagocytosis of Wnt inhibitor SFRP4 by late wound macrophages drives chronic Wnt activity for fibrotic skin healing. Sci Adv 6(12):eaay3704. https://doi.org/10.1126/sciadv.aay3704
Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG (2009) Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci 29(12):3956–3968. https://doi.org/10.1523/JNEUROSCI.3992-08.2009
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29(43):13435–13444. https://doi.org/10.1523/JNEUROSCI.3257-09.2009
Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV (2012) Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33(34):8793–8801. https://doi.org/10.1016/j.biomaterials.2012.08.050
Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ et al (2014) Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci 34(14):4822–4836. https://doi.org/10.1523/JNEUROSCI.4369-13.2014
Jiménez-García L, Herránz S, Luque A, Hortelano S (2015) Thioglycollate-elicited peritoneal macrophages preparation and arginase activity measurement in IL-4 stimulated macrophages. Bio-protocol. https://doi.org/10.21769/BioProtoc.1585
Stein M, Keshav S, Harris N, Gordon S (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176(1):287–292
Endo T, Kadoya K, Suzuki Y, Kawamura D, Iwasaki N (2019) A novel experimental model to determine the axon-promoting effects of grafted cells after peripheral nerve injury. Front Cell Neurosci. https://doi.org/10.3389/fncel.2019.00280
Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT et al (2009) Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 64(2):165–172. https://doi.org/10.1016/j.neuron.2009.09.016
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M (2004) Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58(2):167–176. https://doi.org/10.1002/cyto.a.20022
Endo T, Kadoya K, Kawamura D, Iwasaki N (2019) Evidence for cell-contact factor involvement in neurite outgrowth of DRG neurons stimulated by Schwann cells. Exp Physiol. https://doi.org/10.1113/EP087634
Akula SK, McCullough KB, Weichselbaum C, Dougherty JD, Maloney SE (2020) The trajectory of gait development in mice. Brain Behav 10(6):e01636. https://doi.org/10.1002/brb3.1636
Hirose K, Iwakura N, Orita S, Yamashita M, Inoue G, Yamauchi K et al (2010) Evaluation of behavior and neuropeptide markers of pain in a simple, sciatic nerve-pinch pain model in rats. Eur Spine J 19(10):1746–1752. https://doi.org/10.1007/s00586-010-1428-4
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88. https://doi.org/10.1016/0304-3959(88)90026-7
Nirogi R, Goura V, Shanmuganathan D, Jayarajan P, Abraham R (2012) Comparison of manual and automated filaments for evaluation of neuropathic pain behavior in rats. J Pharmacol Toxicol Methods 66(1):8–13. https://doi.org/10.1016/j.vascn.2012.04.006
Deuis JR, Dvorakova LS, Vetter I (2017) Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10:284. https://doi.org/10.3389/fnmol.2017.00284
Anderson KD, Gunawan A, Steward O (2005) Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp Neurol 194(1):161–174. https://doi.org/10.1016/j.expneurol.2005.02.006
Banik RK, Kabadi RA (2013) A modified Hargreaves’ method for assessing threshold temperatures for heat nociception. J Neurosci Methods 219(1):41–51. https://doi.org/10.1016/j.jneumeth.2013.06.005
Sayanagi J, Tanaka H, Ebara M, Okada K, Oka K, Murase T et al (2020) Combination of electrospun nanofiber sheet incorporating methylcobalamin and PGA-collagen tube for treatment of a sciatic nerve defect in a rat model. J Bone Joint Surg Am 102(3):245–253. https://doi.org/10.2106/JBJS.19.00254
Paul CD, Devine A, Bishop K, Xu Q, Wulftange WJ, Burr H et al (2019) Human macrophages survive and adopt activated genotypes in living zebrafish. Sci Rep 9(1):1759. https://doi.org/10.1038/s41598-018-38186-y
Roszer T (2018) Understanding the biology of self-renewing macrophages. Cells 7(8):103. https://doi.org/10.3390/cells7080103
Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA et al (1994) Nerve crush injuries—a model for axonotmesis. Exp Neurol 127(2):284–290. https://doi.org/10.1006/exnr.1994.1104
Chen LE, Seaber AV, Glisson RR, Davies H, Murrell GA, Anthony DC et al (1992) The functional recovery of peripheral nerves following defined acute crush injuries. J Orthop Res 10(5):657–664. https://doi.org/10.1002/jor.1100100508
Perkins JR, Antunes-Martins A, Calvo M, Grist J, Rust W, Schmid R et al (2014) A comparison of RNA-seq and exon arrays for whole genome transcription profiling of the L5 spinal nerve transection model of neuropathic pain in the rat. Mol Pain 10:7. https://doi.org/10.1186/1744-8069-10-7
Gerrick KY, Gerrick ER, Gupta A, Wheelan SJ, Yegnasubramanian S, Jaffee EM (2018) Transcriptional profiling identifies novel regulators of macrophage polarization. PLoS One 13(12):e0208602. https://doi.org/10.1371/journal.pone.0208602
Siqueira Mietto B, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S (2015) Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J Neurosci 35(50):16431–16442. https://doi.org/10.1523/JNEUROSCI.2119-15.2015
Siconolfi LB, Seeds NW (2001) Induction of the plasminogen activator system accompanies peripheral nerve regeneration after sciatic nerve crush. J Neurosci 21(12):4336–4347
Siconolfi LB, Seeds NW (2001) Mice lacking tPA, uPA, or plasminogen genes showed delayed functional recovery after sciatic nerve crush. J Neurosci 21(12):4348–4355. https://doi.org/10.1523/Jneurosci.21-12-04348.2001
Merino P, Diaz A, Jeanneret V, Wu F, Torre E, Cheng L et al (2017) Urokinase-type plasminogen activator (uPA) binding to the uPA receptor (uPAR) promotes axonal regeneration in the central nervous system. J Biol Chem 292(7):2741–2753. https://doi.org/10.1074/jbc.M116.761650
Lino N, Fiore L, Rapacioli M, Teruel L, Flores V, Scicolone G et al (2014) uPA-uPAR molecular complex is involved in cell signaling during neuronal migration and neuritogenesis. Dev Dyn 243(5):676–689. https://doi.org/10.1002/dvdy.24114
Merino P, Yepes M (2018) Urokinase-type plasminogen activator induces neurorepair in the ischemic brain. J Neurol Exp Neurosci 4(2):24–29. https://doi.org/10.17756/jnen.2018-039
Klimovich PS, Semina EV, Karagyaur MN, Rysenkova KD, Sysoeva VY, Mironov NA et al (2020) Urokinase receptor regulates nerve regeneration through its interaction with alpha5beta1-integrin. Biomed Pharmacother 125:110008. https://doi.org/10.1016/j.biopha.2020.110008
Smith HW, Marshall CJ (2010) Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11(1):23–36. https://doi.org/10.1038/nrm2821
Landowski LM, Pavez M, Brown LS, Gasperini R, Taylor BV, West AK et al (2016) Low-density lipoprotein receptor-related proteins in a novel mechanism of axon guidance and peripheral nerve regeneration. J Biol Chem 291(3):1092–1102. https://doi.org/10.1074/jbc.M115.668996
Rysenkova KD, Klimovich PS, Shmakova AA, Karagyaur MN, Ivanova KA, Aleksandrushkina NA et al (2020) Urokinase receptor deficiency results in EGFR-mediated failure to transmit signals for cell survival and neurite formation in mouse neuroblastoma cells. Cell Signal 75:109741. https://doi.org/10.1016/j.cellsig.2020.109741
Saleh A, Smith DR, Tessler L, Mateo AR, Martens C, Schartner E et al (2013) Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol 249:149–159. https://doi.org/10.1016/j.expneurol.2013.08.018
Tanabe K, Bonilla I, Winkles JA, Strittmatter SM (2003) Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci 23(29):9675–9686
Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM et al (2014) Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83(2):331–343. https://doi.org/10.1016/j.neuron.2014.06.016
van Beek AA, Van den Bossche J, Mastroberardino PG, de Winther MPJ, Leenen PJM (2019) Metabolic alterations in aging macrophages: ingredients for inflammaging? Trends Immunol 40(2):113–127. https://doi.org/10.1016/j.it.2018.12.007
Ma CH, Omura T, Cobos EJ, Latremoliere A, Ghasemlou N, Brenner GJ et al (2011) Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest 121(11):4332–4347. https://doi.org/10.1172/JCI58675
Sakuma M, Gorski G, Sheu SH, Lee S, Barrett LB, Singh B et al (2016) Lack of motor recovery after prolonged denervation of the neuromuscular junction is not due to regenerative failure. Eur J Neurosci 43(3):451–462. https://doi.org/10.1111/ejn.13059
Acknowledgements
We thank M. Endo for their assistance with immunohistochemistry. We are thankful to the National BioResource Project-Rat for providing LEWTg (CAG-EGFP) rats, the Genome Information Research Center for providing Sprague Dawley-Tg (CAG-EGFP) rats, and the Open Facility, Hokkaido University Sousei Hall for access to the cryostat.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (17K11527), the Japan Agency for Medical Research and Development (JP20gm6210004), the General Insurance Association of Japan (2017–2020), the Kobayashi Foundation, and the SEI Group CSR Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: YM and KK. Methodology: YM, KK and AT. Investigation: YM, KK, YN, MH, GM, TS, YY and AT. Funding acquisition: KK and NI. Project administration: KK and NI. Supervision: KK and NI. Writing—original draft: YM and KK.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experimental procedures involving animals were approved by the local ethical committee of the Hokkaido University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2022_4310_MOESM1_ESM.jpg
Suppl. Fig. 1: Gait analysis of the graft of IL-4Mφ after PNI The gait analysis was performed at pre-injury, and at 4 and 8 weeks after the crush injuries using DigiGait. No statistical difference was detected between the rats injected with the IL-4Mφ graft and the control rats in most of the gait parameters except paw drag and swing duration at 8 weeks after injury. *P < 0.05 vs. PBS injection. Student’s t test was used for analysis. N = 10 per group. Error bars represent SEM. (JPG 150 KB)
Rights and permissions
About this article
Cite this article
Matsui, Y., Kadoya, K., Nagano, Y. et al. IL4 stimulated macrophages promote axon regeneration after peripheral nerve injury by secreting uPA to stimulate uPAR upregulated in injured axons. Cell. Mol. Life Sci. 79, 289 (2022). https://doi.org/10.1007/s00018-022-04310-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04310-5