Abstract
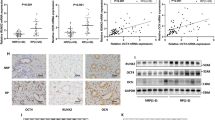
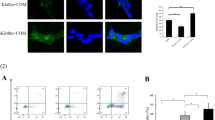
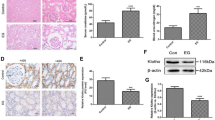
Randall’s plaques (RP) are well established as precursor lesions of idiopathic calcium oxalate (CaOx) stones, and the process of biomineralization driven by osteogenic-like cells has been highlighted in RP formation, but the mechanism is poorly understood. Given the inhibitory role of α-Klotho (KL), an aging suppressor protein with high expression in kidneys, in ectopic calcification and the close association between KL gene polymorphisms and urolithiasis susceptibility, we determined the potential role of KL in RP formation. This study found that both soluble KL (s-KL) and transmembrane KL (m-KL) were downregulated, and that s-KL but not m-KL was inversely correlated with upregulation of osteogenic markers in RP tissues. Additionally, s-KL expression was markedly suppressed in human renal interstitial fibroblasts (hRIFs) and slightly suppressed in HK-2 cells after osteogenic induction, intriguingly, which was echoed to the greater osteogenic capability of hRIFs than HK-2 cells. Further investigations showed the inhibitory effect of s-KL on hRIF osteogenic differentiation in vitro and in vivo. Moreover, coculture with recombinant human KL (r-KL) or HK-2 cells suppressed osteogenic differentiation of hRIFs, and this effect was abolished by coculture with KL-silenced HK-2 cells or the β-catenin agonist SKL2001. Mechanistically, s-KL inactivated the Wnt–β-catenin pathway by directly binding to Wnt2 and upregulating SFRP1. Further investigations identified activation of the Wnt–β-catenin pathway and downregulation of SFRP1 and DKK1 in RP tissues. In summary, this study identified s-KL deficiency as a pathological feature of RP and revealed that s-KL released from HK-2 cells inhibited osteogenic differentiation of hRIFs by inactivating the Wnt–β-catenin pathway, not only providing in-depth insight into the role of s-KL in renal interstitial biomineralization but also shedding new light on the interaction of renal tubular epithelial cells with interstitial cells to clarify RP formation.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- ARS:
-
Alizarin Red staining
- ALP:
-
Alkaline phosphatase
- CaOx:
-
Calcium oxalate
- CaP:
-
Calcium phosphate
- CaOx-SF:
-
CaOx stone formers
- HC:
-
Healthy controls
- hRIFs:
-
Human renal interstitial fibroblasts
- KL :
-
KLOTHO
- NRP:
-
Normal renal papillae
- RP:
-
Randall’s plaques
- r-KL:
-
Recombinant human KL
- SFRPs:
-
Secreted Frizzled-related proteins
- s-KL:
-
Soluble α-Klotho
- m-KL:
-
Transmembrane α-Klotho
- UKCR:
-
Urinary s-KL-to-creatinine ratio
References
Turney BW, Reynard JM, Noble JG and Keoghane SR (2012) Trends in urological stone disease. BJU Int 109: 1082-+. DOI: https://doi.org/10.1111/j.1464-410X.2011.10495.x.
Neisius A, Preminger GM (2013) Epidemiology, prevention and redefining therapeutic standards. Nat Rev Urol 10:75–77. https://doi.org/10.1038/nrurol.2012.253
Heers H, Turney BW (2016) Trends in urological stone disease: a 5-year update of hospital episode statistics. BJU Int 118:785–789. https://doi.org/10.1111/bju.13520
Canales BK, Hatch M (2014) Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Related Dis 10:734–742. https://doi.org/10.1016/j.soard.2014.03.026
Evan AP (2010) Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatric Nephrol (Berlin, Germany) 25:831–841. https://doi.org/10.1007/s00467-009-1116-y
Darves-Bornoz A, Marien T, Thomas J et al (2019) Renal papillary mapping and quantification of randall’s plaque in pediatric calcium oxalate stone formers. J Endourol 33:863–867. https://doi.org/10.1089/end.2019.0377
Randall A (1937) The origin and growth of renal calculi. Annals Surg 105:1009–1027. https://doi.org/10.1097/00000658-193706000-00014
Khan SR, Canales BK (2015) Unified theory on the pathogenesis of Randall’s plaques and plugs. Urolithiasis 43(Suppl 1):109–123. https://doi.org/10.1007/s00240-014-0705-9
Evan AP, Worcester EM, Coe FL, Williams J Jr, Lingeman JE (2015) Mechanisms of human kidney stone formation. Urolithiasis 43(Suppl 1):19–32. https://doi.org/10.1007/s00240-014-0701-0
Priante G, Ceol M, Gianesello L et al (2019) Human proximal tubular cells can form calcium phosphate deposits in osteogenic culture: role of cell death and osteoblast-like transdifferentiation. Cell death discovery 5:57. https://doi.org/10.1038/s41420-019-0138-x
Gay C, Letavernier E, Verpont MC et al (2020) Nanoscale analysis of randall’s plaques by electron energy loss spectromicroscopy: insight in early biomineral formation in human kidney. ACS Nano 14:1823–1836. https://doi.org/10.1021/acsnano.9b07664
Khan SR and Gambaro G (2016) Role of osteogenesis in the formation of randall's plaques. Anatomical record (Hoboken, NJ : 2007) 299: 5–7. DOI: https://doi.org/10.1002/ar.23275.
Kuro-o M, Matsumura Y, Aizawa H et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Xu Y, Sun Z (2015) Molecular basis of Klotho: from gene to function in aging. Endocrine Rev 36:174–193. https://doi.org/10.1210/er.2013-1079
Lindberg K, Amin R, Moe OW et al (2014) The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25:2169–2175. https://doi.org/10.1681/asn.2013111209
Li SA, Watanabe M, Yamada H et al (2004) Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29:91–99. https://doi.org/10.1247/csf.29.91
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104:19796–19801. https://doi.org/10.1073/pnas.0709805104
Imura A, Iwano A, Tohyama O et al (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565:143–147. https://doi.org/10.1016/j.febslet.2004.03.090
Mencke R, Harms G, Moser J, et al (2017) Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI insight 2. DOI: https://doi.org/10.1172/jci.insight.94375
Saar-Kovrov V, Donners M, van der Vorst EPC (2020) Shedding of klotho: functional implications in chronic kidney disease and associated vascular disease. Front Cardiovasc Med 7:617842. https://doi.org/10.3389/fcvm.2020.617842
Dalton GD, Xie J, An SW, Huang CL (2017) New insights into the mechanism of action of soluble klotho. Front Endocrinol 8:323. https://doi.org/10.3389/fendo.2017.00323
Kurosu H, Yamamoto M, Clark JD et al (2005) Suppression of aging in mice by the hormone Klotho. Science (New York, NY) 309:1829–1833. https://doi.org/10.1126/science.1112766
Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M (2005) Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126:1274–1283. https://doi.org/10.1016/j.mad.2005.07.007
Li F, Yao Q, Ao L et al (2017) Klotho suppresses high phosphate-induced osteogenic responses in human aortic valve interstitial cells through inhibition of Sox9. J Mol Med (Berlin, Germany) 95:739–751. https://doi.org/10.1007/s00109-017-1527-3
Zhang W, Xue D, Hu D et al (2015) Secreted klotho protein attenuates osteogenic differentiation of human bone marrow mesenchymal stem cells in vitro via inactivation of the FGFR1/ERK signaling pathway. Growth factors (Chur, Switzerland) 33:356–365. https://doi.org/10.3109/08977194.2015.1108313
Zhu Z, Xia W, Cui Y et al (2019) Klotho gene polymorphisms are associated with healthy aging and longevity: evidence from a meta-analysis. Mech Ageing Dev 178:33–40. https://doi.org/10.1016/j.mad.2018.12.003
Zhou L, Li Y, Zhou D, Tan RJ, Liu Y (2013) Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24:771–785. https://doi.org/10.1681/asn.2012080865
Evan AP, Coe FL, Lingeman J, Bledsoe S, Worcester EM (2018) Randall’s plaque in stone formers originates in ascending thin limbs. Am J Physiol Renal Physiol 315:F1236-f1242. https://doi.org/10.1152/ajprenal.00035.2018
Zhu Z, Huang F, Xia W et al (2020) Osteogenic differentiation of renal interstitial fibroblasts promoted by lncRNA MALAT1 may partially contribute to randall’s plaque formation. Front Cell Dev Biol 8:596363. https://doi.org/10.3389/fcell.2020.596363
Huang Y, Zheng Y, Jia L, Li W (2015) Long noncoding RNA H19 promotes osteoblast differentiation via TGF-beta1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cells (Dayton, Ohio) 33:3481–3492. https://doi.org/10.1002/stem.2225
Yamazaki Y, Imura A, Urakawa I et al (2010) Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398:513–518. https://doi.org/10.1016/j.bbrc.2010.06.110
Shen K, Vesey DA, Ellis RJ et al (2019) GRP78 expression in tumor and perinephric adipose tissue is not an optimal risk stratification marker for clear cell renal cell carcinoma. PLoS ONE 14:e0210246. https://doi.org/10.1371/journal.pone.0210246
Shu J, Dolman GE, Duan J, Qiu G, Ilyas M (2016) Statistical colour models: an automated digital image analysis method for quantification of histological biomarkers. Biomed Eng Online 15:46. https://doi.org/10.1186/s12938-016-0161-6
Liu H, Fergusson MM, Castilho RM et al (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science (New York, NY) 317:803–806. https://doi.org/10.1126/science.1143578
Li H, Zhou J, Zhu M et al (2020) Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J Biomed Materials Res Part A. https://doi.org/10.1002/jbm.a.37102
Pillai ICL, Li S, Romay M et al (2017) Cardiac fibroblasts adopt osteogenic fates and can be targeted to attenuate pathological heart calcification. Cell Stem Cell 20(218–232):e215. https://doi.org/10.1016/j.stem.2016.10.005
Hu MC, Shi M, Zhang J et al (2016) Renal Production, Uptake, and Handling of Circulating αKlotho. J Am Soc Nephrol 27:79–90. https://doi.org/10.1681/asn.2014101030
Khan SR (2015) The role of Randall’s plaques in urolithiasis. Foreword Urolithiasis 43(Suppl 1):1–3. https://doi.org/10.1007/s00240-014-0721-9
Houschyar KS, Tapking C, Borrelli MR et al (2018) Wnt pathway in bone repair and regeneration—What Do We Know So Far. Front Cell Dev Biol 6:170. https://doi.org/10.3389/fcell.2018.00170
Chen D, Xie R, Shu B et al (2019) Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann New York Acad Sci 1442:48–60. https://doi.org/10.1111/nyas.13945
Carrillo-López N, Panizo S, Alonso-Montes C et al (2016) Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int 90:77–89. https://doi.org/10.1016/j.kint.2016.01.024
Thongprayoon C, Krambeck AE, Rule AD (2020) Determining the true burden of kidney stone disease. Nat Rev Nephrol 16:736–746. https://doi.org/10.1038/s41581-020-0320-7
Chen J, Lin Y, Sun Z (2016) Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKα-mediated activation of RUNX2. Aging Cell 15:853–860. https://doi.org/10.1111/acel.12494
Gu Y, Ren K, Wang L, Yao Q (2019) Loss of Klotho contributes to cartilage damage by derepression of canonical Wnt/β-catenin signaling in osteoarthritis mice. Aging 11:12793–12809. https://doi.org/10.18632/aging.102603
Zhu Z, Cui Y, Huang F et al (2020) Long non-coding RNA H19 promotes osteogenic differentiation of renal interstitial fibroblasts through Wnt-β-catenin pathway. Mol Cell Biochem 470:145–155. https://doi.org/10.1007/s11010-020-03753-3
Liu Q, Zhu LJ, Waaga-Gasser AM et al (2019) The axis of local cardiac endogenous Klotho-TGF-β1-Wnt signaling mediates cardiac fibrosis in human. J Mol Cell Cardiol 136:113–124. https://doi.org/10.1016/j.yjmcc.2019.09.004
Miao J, Liu J, Niu J et al (2019) Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18:e13004. https://doi.org/10.1111/acel.13004
Khan SR, Canales BK, Dominguez-Gutierrez PR (2021) Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat Rev Nephrol. https://doi.org/10.1038/s41581-020-00392-1
Hu MC, Shi M, Moe OW (2019) Role of alphaKlotho and FGF23 in regulation of type II Na-dependent phosphate co-transporters. Pflugers Arch 471:99–108. https://doi.org/10.1007/s00424-018-2238-5
Kuro OM (2019) The Klotho proteins in health and disease. Nat Rev Nephrol 15:27–44. https://doi.org/10.1038/s41581-018-0078-3
Melo TL, Esper PLG, Zambrano LI et al (2020) Expression of vitamin D receptor, CYP27B1 and CYP24A1 hydroxylases and 1,25-dihydroxyvitamin D(3) levels in stone formers. Urolithiasis 48:19–26. https://doi.org/10.1007/s00240-019-01163-9
Grange C, Papadimitriou E, Dimuccio V et al (2020) Urinary Extracellular Vesicles Carrying Klotho Improve the Recovery of Renal Function in an Acute Tubular Injury Model. Mol Therapy 28:490–502. https://doi.org/10.1016/j.ymthe.2019.11.013
Wang N, Ma J, Ren Y, Xiang S, Jia R (2019) Urinary extracellular vesicles carrying klotho improve the recovery of renal function in an acute tubular injury model. Am J Trans Res 11:3375–3383
Wu XR (2015) Interstitial calcinosis in renal papillae of genetically engineered mouse models: relation to Randall’s plaques. Urolithiasis 43(Suppl 1):65–76. https://doi.org/10.1007/s00240-014-0699-3
Khan SR, Canales BK (2011) Ultrastructural investigation of crystal deposits in Npt2a knockout mice: are they similar to human Randall’s plaques? J Urol 186:1107–1113. https://doi.org/10.1016/j.juro.2011.04.109
Acknowledgements
Thank National Natural Science Foundation of China, Natural Science Foundation of Hunan Province for providing funding for this study and Central South University Independent Exploration and Innovation Project for Graduate Students.
Funding
This work was supported by the National Natural Science Foundation of China (81770705 to Hequn Chen; 82000761 to Yu Cui); Natural Science Foundation of Hunan Province (2019JJ40488 to Zhiyong Chen); Central South University Independent Exploration and Innovation Project for Graduate Students (2021zzts0348 to Zewu Zhu).
Author information
Authors and Affiliations
Contributions
ZZ, ZC and HC performed study concept and designed the experiment. ZZ, SR, YJ, FH, WX, JC, YC, CH and FZ contributed to the development of methodology and the acquisition of data. ZZ, YL and ZC contributed to analysis and interpretation of data. ZZ, ZC and HC contributed to writing and revision of the manuscript. ZZ, YC and HC contributed to the administrative, technical and material support. ZC and HC contributed to study supervision. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval
Approval was granted by Xiangya Hospital Ethics Committee (Approval Number: 202103089) and the Institutional Experimental Animal Committee of Central South University (Proof Number: 2021sydw0033).
Consent to participate
Written informed consents for surgical procedures and getting samples were obtained from all included patients prior to surgery. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Written informed consents for publishing clinical characteristics on the condition of anonymity were obtained from all included patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2021_3972_MOESM1_ESM.tif
Supplementary file1. Supplementary Figure 1. 2% agarose gel electrophoresis was performed to verify the PCR products amplified by each pair of primers (TIF 8167 KB)
18_2021_3972_MOESM2_ESM.tif
Supplementary file2. Supplementary Figure 2. (A) The DAB staining density of soluble α-Klotho (s-KL) and osteogenic markers (Runx2, MSX2, OCN) was quantified by average gray value using the IHC Toolbox plugin in ImageJ (Randall’s Plaques (RP), n=6; normal renal papillae (NRP), n=6). (B–D) Correlation analysis between the relative protein expression levels of s-KL and transmembrane KL (m-KL), Runx2, and OCN in RP tissues; n=18. (E–G) Correlation analysis between the relative protein expression levels of m-KL and osteogenic markers (Runx2, MSX2, and OCN) in RP tissues; n=18. (H–K) Quantification of s-KL and osteogenic marker expression determined by WB in human renal interstitial fibroblasts (hRIFs) 1, 4, 7, and 14 days after culture in either osteogenic medium (OM) or normal medium (NM); n=3. (L–O) Quantification of s-KL and osteogenic marker expression determined by WB in HK-2 cells 1, 4, 7, and 14 days after culture in either OM or NM; n=3 (TIF 515 KB)
18_2021_3972_MOESM3_ESM.tif
Supplementary file3. Supplementary Figure 3. (A–B) qRT-PCR was used to determine the mRNA expression level of α-Klotho (KL) in human interstitial fibroblasts (hRIFs) transfected with Len-ctrl, Len-KL, Len-sh-ctrl, Len-sh1-KL, Len-sh2-KL, and Len-sh3-KL; n=3. Len-sh-KL-3, with the highest efficiency, was used to silence KL in hRIFs in the following experiments. (C) qRT-PCR was used to determine the relative mRNA expression level of KL in hRIFs and HK-2 cells; n=3. (D–E) Western blots and quantitative analysis of soluble α-Klotho (s-KL) and transmembrane KL (m-KL) in hRIFs and HK-2 cells; n=3. (F–G) qRT-PCR was used to determine the mRNA expression level of KL in HK-2 cells transfected with Len-ctrl, Len-KL, Len-sh-ctrl, Len-sh1-KL, Len-sh2-KL, and Len-sh3-KL; n=3. Len-sh-KL-1, with the highest efficiency, was used to silence KL in HK-2 cells in the following experiments. (H) Representative western blots of Wnt9a in whole-cell lysates of hRIFs and coimmunoprecipitation with the anti-s-KL antibody, IgG or unconjugated beads as a negative control (NC); n=3. Immunoblotting=IB; immunoprecipitation=IP. (I) Representative western blots of Wnt5b in whole-cell lysates of hRIFs and coimmunoprecipitation with the anti-s-KL antibody, IgG or unconjugated beads; n=3. (J) Quantitative analysis of the western blot results for SFRP1 and SFRP4 in hRIFs retransfected with siRNAs targeting SFRP1 and induced with OM for 7 days; n=3. (K) Quantitative analysis of the western blot results for SFRP1 and SFRP4 in hRIFs retransfected with siRNAs targeting SFRP4 and induced with OM for 7 days; n=3 (TIF 10454 KB)
18_2021_3972_MOESM4_ESM.tif
Supplementary file4. Supplementary Figure 4. (A–B) Correlation analysis between the relative protein expression levels of SFRP1 and MSX2 and OCN in RP (Randall’s plaque) tissues; n=18. (C–D) Correlation analysis between the relative protein expression levels of DKK1 and Runx2 and MSX2 in RP tissues; n=18. (E–I) Correlation analysis between the ratio of p-β-catenin to β-catenin and the relative protein expression levels of SFRP1, DKK1, Runx2, MSX2, and OCN in RP tissues; n=18. (J–L) Correlation analysis between the relative protein expression level of transmembrane α-Klotho (m-KL) and the ratio of p-β-catenin to β-catenin, SFRP1 expression, and DKK1 expression in RP tissues; n=18 (TIF 8065 KB)
Rights and permissions
About this article
Cite this article
Zhu, Z., Ruan, S., Jiang, Y. et al. α-Klotho released from HK-2 cells inhibits osteogenic differentiation of renal interstitial fibroblasts by inactivating the Wnt–β-catenin pathway. Cell. Mol. Life Sci. 78, 7831–7849 (2021). https://doi.org/10.1007/s00018-021-03972-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03972-x