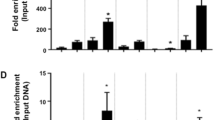
Abstract
Epigenetic modifications play a central role in cell differentiation and development. In the current study, we have recognized lysine demethylase 4A (KDM4A) as a novel epigenetic regulator of osteoblast and adipocyte differentiation. Kdm4a expression was upregulated during osteogenesis and adipogenesis of primary marrow stromal cells and established stromal ST2 line. Overexpression of wild-type Kdm4a promoted adipogenic differentiation and blocked osteogenic differentiation of the progenitor cells. This effect was largely alleviated when the catalytically dead mutation was made. Conversely, depletion or inactivation of Kdm4a in undifferentiated progenitor cells inhibited the formation of adipocytes and promoted the differentiation of osteoblasts. Mechanism explorations showed that overexpression of Kdm4a upregulated the expression of secreted frizzled-related protein 4 (Sfrp4) and CCAAT/enhancer-binding protein α (C/ebpα). Chromatin immunoprecipitation assay demonstrated that KDM4A directly bound the promoters of Sfrp4 and C/ebpα, removed the histone methylation mark H3K9me3, and reduced DNA methylation levels of CpG in promoter regions of C/ebpα and Sfrp4. Furthermore, overexpression of Kdm4a inactivated canonical Wnt signaling. Moreover, activation of canonical Wnt signaling through silencing of Sfrp4 in ST2 attenuated the inhibition of osteogenic differentiation and the enhancement of adipogenic differentiation by KDM4A. These data have identified KDM4A as a novel regulator of osteoblast and adipocyte differentiation and suggest KDM4A inhibition as a potential therapeutic target for treating metabolic disorders such as osteoporosis.










Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- FABP4:
-
Fatty acid binding protein 4
- C/EBP:
-
CCAAT/enhancer-binding protein
- DLX:
-
Distal-less homeobox
- DNMT3B:
-
DNA methyltransferase 3
- HOX:
-
Homeobox
- HP1:
-
Heterochromatin protein 1 (HP1)
- KDM4A:
-
Lysine demethylase 4A
- Lrp6:
-
Low-density lipoprotein receptor-related protein 6
- MSC:
-
Mesenchymal stem cell
- Osx:
-
Osterix
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- Runx2:
-
Runt-related transcription factor 2
- SFRP4:
-
Secreted frizzled-related protein 4
- TCF7L2:
-
Transcription factor 7 like 2
References
Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2(4):313–319
Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH (2009) Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev 18(7):955–968
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2(3):165–171
Chen X, Ayala I, Shannon C, Fourcaudot M, Acharya NK, Jenkinson CP, Heikkinen S, Norton L (2018) The diabetes gene and Wnt pathway effector TCF7L2 regulates adipocyte development and function. Diabetes 67(4):554–568
Sottile V, Seuwen K (2000) Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett 475(3):201–204
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC (2009) A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18(4):545–559
Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, Mishina Y (2018) TGF-beta family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol 10(5):a022202
Siersbaek R, Nielsen R, Mandrup S (2012) Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab 23(2):56–64
Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B (2009) PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106(2):232–246
Chen JC, Chua M, Bellon RB, Jacobs CR (2015) Epigenetic changes during mechanically induced osteogenic lineage commitment. J Biomech Eng 137(2):020902
Arnsdorf EJ, Tummala P, Castillo AB, Zhang F, Jacobs CR (2010) The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomech 43(15):2881–2886
Shi Y (2007) Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet 8(11):829–833
Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park NH, Wang CY (2012) Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell 11(1):50–61
Lee HL, Yu B, Deng P, Wang CY, Hong C (2016) Transforming growth factor-beta-induced KDM4B promotes chondrogenic differentiation of human mesenchymal stem cells. Stem Cells 34(3):711–719
Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y (2006) Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125(3):467–481
Kim TD, Jin F, Shin S, Oh S, Lightfoot SA, Grande JP, Johnson AJ, van Deursen JM, Wren JD, Janknecht R (2016) Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. J Clin Investig 126(2):706–720
Kogure M, Takawa M, Cho HS, Toyokawa G, Hayashi K, Tsunoda T, Kobayashi T, Daigo Y, Sugiyama M, Atomi Y, Nakamura Y, Hamamoto R (2013) Deregulation of the histone demethylase JMJD2A is involved in human carcinogenesis through regulation of the G(1)/S transition. Cancer Lett 336(1):76–84
Mallette FA, Richard S (2012) JMJD2A promotes cellular transformation by blocking cellular senescence through transcriptional repression of the tumor suppressor CHD5. Cell Rep 2(5):1233–1243
Li LL, Xue AM, Li BX, Shen YW, Li YH, Luo CL, Zhang MC, Jiang JQ, Xu ZD, Xie JH, Zhao ZQ (2014) JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI. Breast Cancer Res 16(3):R56
Metzger E, Stepputtis SS, Strietz J, Preca BT, Urban S, Willmann D, Allen A, Zenk F, Iovino N, Bronsert P, Proske A, Follo M, Boerries M, Stickeler E, Xu J, Wallace MB, Stafford JA, Kanouni T, Maurer J, Schule R (2017) KDM4 inhibition targets breast cancer stem-like cells. Cancer Res 77(21):5900–5912
Berry WL, Janknecht R (2013) KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res 73(10):2936–2942
Dobrynin G, McAllister TE, Leszczynska KB, Ramachandran S, Krieg AJ, Kawamura A, Hammond EM (2017) KDM4A regulates HIF-1 levels through H3K9me3. Sci Rep 7(1):11094
Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L, Peters JH, DeMeester SR, DeMeester TR, Skinner KA, Laird PW (2001) Epigenetic patterns in the progression of esophageal adenocarcinoma. Can Res 61(8):3410–3418
Ishiguro K, Watanabe O, Nakamura M, Yamamura T, Matsushita M, Goto H, Hirooka Y (2017) Inhibition of KDM4A activity as a strategy to suppress interleukin-6 production and attenuate colitis induction. Clin Immunol 180:120–127
Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S, Chen T, Li E, Jenuwein T, Peters AH (2003) Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol 13(14):1192–1200
Vincent A, Van Seuningen I (2009) Epigenetics, stem cells and epithelial cell fate. Differentiation 78(2–3):99–107
Lee JE, Ge K (2014) Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci 4:29
Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y (2013) The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell 52(1):101–112
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J (2016) PPARgamma and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther 11(3):216–225
Abdallah BM, Jafari A, Zaher W, Qiu W, Kassem M (2015) Skeletal (stromal) stem cells: an update on intracellular signaling pathways controlling osteoblast differentiation. Bone 70:28–36
Kim HJ, Park JW, Lee KH, Yoon H, Shin DH, Ju UI, Seok SH, Lim SH, Lee ZH, Kim HH, Chun YS (2014) Plant homeodomain finger protein 2 promotes bone formation by demethylating and activating Runx2 for osteoblast differentiation. Cell Res 24(10):1231–1249
Musri MM, Corominola H, Casamitjana R, Gomis R, Parrizas M (2006) Histone H3 lysine 4 dimethylation signals the transcriptional competence of the adiponectin promoter in preadipocytes. J Biol Chem 281(25):17180–17188
Jang MK, Kim JH, Jung MH (2017) Histone H3K9 demethylase JMJD2B activates adipogenesis by regulating H3K9 methylation on PPARgamma and C/EBPalpha during adipogenesis. PLoS One 12(1):e0168185
Wang C, Wang J, Li J, Hu G, Shan S, Li Q, Zhang X (2016) KDM5A controls bone morphogenic protein 2-induced osteogenic differentiation of bone mesenchymal stem cells during osteoporosis. Cell Death Dis 7(8):e2335
Yu G, Wang J, Lin X, Diao S, Cao Y, Dong R, Wang L, Wang S, Fan Z (2016) Demethylation of SFRP2 by histone demethylase KDM2A regulated osteo-/dentinogenic differentiation of stem cells of the apical papilla. Cell Prolif 49(3):330–340
Yang X, Wang G, Wang Y, Zhou J, Yuan H, Li X, Liu Y, Wang B (2019) Histone demethylase KDM7A reciprocally regulates adipogenic and osteogenic differentiation via regulation of C/EBPalpha and canonical Wnt signalling. J Cell Mol Med 23(3):2149–2162
Matsumura Y, Nakaki R, Inagaki T, Yoshida A, Kano Y, Kimura H, Tanaka T, Tsutsumi S, Nakao M, Doi T, Fukami K, Osborne TF, Kodama T, Aburatani H, Sakai J (2015) H3K4/H3K9me3 bivalent chromatin domains targeted by lineage-specific DNA methylation pauses adipocyte differentiation. Mol Cell 60(4):584–596
Berdasco M, Melguizo C, Prados J, Gomez A, Alaminos M, Pujana MA, Lopez M, Setien F, Ortiz R, Zafra I, Aranega A, Esteller M (2012) DNA methylation plasticity of human adipose-derived stem cells in lineage commitment. Am J Pathol 181(6):2079–2093
Villagra A, Gutierrez J, Paredes R, Sierra J, Puchi M, Imschenetzky M, Wijnen Av A, Lian J, Stein G, Stein J, Montecino M (2002) Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J Cell Biochem 85(1):112–122
Du J, Johnson LM, Jacobsen SE, Patel DJ (2015) DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol 16(9):519–532
Zhao Q, Zhang J, Chen R, Wang L, Li B, Cheng H, Duan X, Zhu H, Wei W, Li J, Wu Q, Han JD, Yu W, Gao S, Li G, Wong J (2016) Dissecting the precise role of H3K9 methylation in crosstalk with DNA maintenance methylation in mammals. Nat Commun 7:12464
Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA (2007) Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem 282(19):14515–14524
Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA (2002) Regulation of Wnt signaling during adipogenesis. J Biol Chem 277(34):30998–31004
Nemoto E, Sakisaka Y, Tsuchiya M, Tamura M, Nakamura T, Kanaya S, Shimonishi M, Shimauchi H (2016) Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res 51(2):164–174
Acknowledgements
The work was funded by National Natural Science Foundation of China (Grants nos. 81871741, 81672116 and 81772297), Natural Science Foundation of Tianjin City Municipal Science and Technology Commission (Grants nos. 18JCZDJC32200 and 18JCQNJC12900) and by Graduate student innovation fund of Tianjin Medical University (Grant no. YJSCX201803).
Author information
Authors and Affiliations
Contributions
QQ, YW, XW, JY, YX and JZ: collection and assembly of data, data analysis and interpretation, and final approval of manuscript; XL: conception and design and final approval of manuscript; BW: conception and design, manuscript writing, and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qi, Q., Wang, Y., Wang, X. et al. Histone demethylase KDM4A regulates adipogenic and osteogenic differentiation via epigenetic regulation of C/EBPα and canonical Wnt signaling. Cell. Mol. Life Sci. 77, 2407–2421 (2020). https://doi.org/10.1007/s00018-019-03289-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03289-w