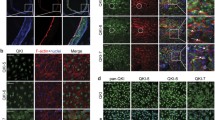
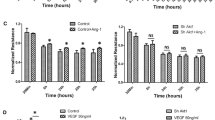
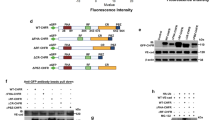
Abstract
VE-cadherin plays a central role in controlling endothelial barrier function, which is transiently disrupted by proinflammatory cytokines such as tumor necrosis factor (TNFα). Here we show that human endothelial cells compensate VE-cadherin degradation in response to TNFα by inducing VE-cadherin de novo synthesis. This compensation increases adherens junction turnover but maintains surface VE-cadherin levels constant. NF-κB inhibition strongly reduced VE-cadherin expression and provoked endothelial barrier collapse. Bacterial lipopolysaccharide and TNFα upregulated the transcription factor ETS1, in vivo and in vitro, in an NF-κB dependent manner. ETS1 gene silencing specifically reduced VE-cadherin protein expression in response to TNFα and exacerbated TNFα-induced barrier disruption. We propose that TNFα induces not only the expression of genes involved in increasing permeability to small molecules and immune cells, but also a homeostatic transcriptional program in which NF-κB- and ETS1-regulated VE-cadherin expression prevents the irreversible damage of endothelial barriers.







Similar content being viewed by others
References
Fernandez-Martin L, Marcos-Ramiro B, Bigarella CL, Graupera M, Cain RJ, Reglero-Real N, Jimenez A, Cernuda-Morollon E, Correas I, Cox S, Ridley AJ, Millan J (2012) Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol 32:e90–e102
Marcos-Ramiro B, Garcia-Weber D, Millan J (2014) TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost 112(6):1088–1102
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Colas-Algora N, Millan J (2019) How many cadherins do human endothelial cells express? Cell Mol Life Sci 76(7):1299–1317
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–157
Giannotta M, Trani M, Dejana E (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26:441–454
Hayer A, Shao L, Chung M, Joubert LM, Yang HW, Tsai FC, Bisaria A, Betzig E, Meyer T (2016) Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat Cell Biol 18:1311–1323
Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, Passaniti A, Hasday JD, Goldblum SE (2006) TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol 291:L1232–L1245
Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB (2014) Evidence of a common mechanism of disassembly of adherens junctions through Galpha13 targeting of VE-cadherin. J Exp Med 211:579–591
Flemming S, Burkard N, Renschler M, Vielmuth F, Meir M, Schick MA, Wunder C, Germer CT, Spindler V, Waschke J, Schlegel N (2015) Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovasc Res 107:32–44
Seynhaeve AL, Rens JA, Schipper D, Eggermont AM, Ten Hagen TL (2014) Exposing endothelial cells to tumor necrosis factor-alpha and peripheral blood mononuclear cells damage endothelial integrity via interleukin-1ss by degradation of vascular endothelial-cadherin. Surgery 155:545–553
Cain RJ, Vanhaesebroeck B, Ridley AJ (2010) The PI3 K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol 188:863–876
Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, Correas I, Ridley AJ (2010) Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol 8:11
Marcos-Ramiro B, Garcia-Weber D, Barroso S, Feito J, Ortega MC, Cernuda-Morollon E, Reglero-Real N, Fernandez-Martin L, Duran MC, Alonso MA, Correas I, Cox S, Ridley AJ, Millan J (2016) RhoB controls endothelial barrier recovery by inhibiting Rac1 trafficking to the cell border. J Cell Biol 213:385–402
Reglero-Real N, Alvarez-Varela A, Cernuda-Morollon E, Feito J, Marcos-Ramiro B, Fernandez-Martin L, Gomez-Lechon MJ, Muntane J, Sandoval P, Majano PL, Correas I, Alonso MA, Millan J (2014) Apicobasal polarity controls lymphocyte adhesion to hepatic epithelial cells. Cell Rep 8:1879–1893
Gavard J, Gutkind JS (2006) VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8:1223–1234
Navarro P, Ruco L, Dejana E (1998) Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140:1475–1484
Aranda JF, Reglero-Real N, Marcos-Ramiro B, Ruiz-Saenz A, Fernandez-Martin L, Bernabe-Rubio M, Kremer L, Ridley AJ, Correas I, Alonso MA, Millan J (2013) MYADM controls endothelial barrier function through ERM-dependent regulation of ICAM-1 expression. Mol Biol Cell 24:483–494
Patel DM, Dubash AD, Kreitzer G, Green KJ (2014) Disease mutations in desmoplakin inhibit Cx43 membrane targeting mediated by desmoplakin-EB1 interactions. J Cell Biol 206:779–797
Toret CP, Collins C, Nelson WJ (2014) An Elmo-Dock complex locally controls Rho GTPases and actin remodeling during cadherin-mediated adhesion. J Cell Biol 207:577–587
Giaever I, Keese CR (1991) Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci USA 88:7896–7900
Colas-Algora N, Millan J (2019) How many cadherins do human endothelial cells express? Cell Mol Life Sci 76:1299–1317
Kruse K, Lee QS, Sun Y, Klomp J, Yang X, Huang F, Sun MY, Zhao S, Hong Z, Vogel SM, Shin JW, Leckband DE, Tai LM, Malik AB, Komarova YA (2019) N-cadherin signaling via Trio assembles adherens junctions to restrict endothelial permeability. J Cell Biol 218:299–316
Kim MS, Lee CS, Hur J, Cho HJ, Jun SI, Kim TY, Lee SW, Suh JW, Park KW, Lee HY, Kang HJ, Lee DS, Koh GY, Nakagami H, Morishita R, Park YB, Kim HS (2009) Priming with angiopoietin-1 augments the vasculogenic potential of the peripheral blood stem cells mobilized with granulocyte colony-stimulating factor through a novel Tie2/Ets-1 pathway. Circulation 120:2240–2250
Arderiu G, Pena E, Aledo R, Espinosa S, Badimon L (2012) Ets-1 transcription is required in tissue factor driven microvessel formation and stabilization. Angiogenesis 15:657–669
Feng W, Xing D, Hua P, Zhang Y, Chen YF, Oparil S, Jaimes EA (2010) The transcription factor ETS-1 mediates proinflammatory responses and neointima formation in carotid artery endoluminal vascular injury. Hypertension 55:1381–1388
Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE (2001) TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis. 159:93–101
Redlich K, Kiener HP, Schett G, Tohidast-Akrad M, Selzer E, Radda I, Stummvoll GH, Steiner CW, Groger M, Bitzan P, Zenz P, Smolen JS, Steiner G (2001) Overexpression of transcription factor Ets-1 in rheumatoid arthritis synovial membrane: regulation of expression and activation by interleukin-1 and tumor necrosis factor alpha. Arthritis Rheum 44:266–274
Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS (2002) Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery 132:180–185
Su W, Kowalczyk AP (2017) The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Mol Biol Cell 28:76–84
Iurlaro M, Demontis F, Corada M, Zanetta L, Drake C, Gariboldi M, Peiro S, Cano A, Navarro P, Cattelino A, Tognin S, Marchisio PC, Dejana E (2004) VE-cadherin expression and clustering maintain low levels of survivin in endothelial cells. Am J Pathol 165:181–189
Yan Z, Wang ZG, Segev N, Hu S, Minshall RD, Dull RO, Zhang M, Malik AB, Hu G (2016) Rab11a mediates vascular endothelial-cadherin recycling and controls endothelial barrier function. Arterioscler Thromb Vasc Biol 36:339–349
Yang J, Yao W, Qian G, Wei Z, Wu G, Wang G (2015) Rab5-mediated VE-cadherin internalization regulates the barrier function of the lung microvascular endothelium. Cell Mol Life Sci 72:4849–4866
Chichger H, Duong H, Braza J, Harrington EO (2015) p18, a novel adaptor protein, regulates pulmonary endothelial barrier function via enhanced endocytic recycling of VE-cadherin. FASEB J 29:868–881
Frye M, Dierkes M, Kuppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D (2015) Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med 212:2267–2287
Hagerling R, Hoppe E, Dierkes C, Stehling M, Makinen T, Butz S, Vestweber D, Kiefer F (2018) Distinct roles of VE-cadherin for development and maintenance of specific lymph vessel beds. EMBO J 37:e98271
Lelievre E, Mattot V, Huber P, Vandenbunder B, Soncin F (2000) ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE-cadherin. Oncogene 19:2438–2446
Wong MM, Chen Y, Margariti A, Winkler B, Campagnolo P, Potter C, Hu Y, Xu Q (2014) Macrophages control vascular stem/progenitor cell plasticity through tumor necrosis factor-alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol 34:635–643
Winsauer G, de Martin R (2007) Resolution of inflammation: intracellular feedback loops in the endothelium. Thromb Haemost 97:364–369
Mahmood T, Yang PC (2012) Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4:429–434
Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I (1992) Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci USA 89:7919–7923
Rivas V, Carmona R, Munoz-Chapuli R, Mendiola M, Nogues L, Reglero C, Miguel-Martin M, Garcia-Escudero R, Dorn GW 2nd, Hardisson D, Mayor F Jr, Penela P (2013) Developmental and tumoral vascularization is regulated by G protein-coupled receptor kinase 2. J Clin Invest 123:4714–4730
Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM (2002) Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 8:27–34
Acknowledgements
The expert technical advice of the Confocal Microscopy Facility and the Genomic Facility are gratefully acknowledged. The work was supported by Grants SAF2017-88187-R and S2017/BMD-3817 TomoXliver (to J.M.), BFU2015–67266-R (to I.C) and Instituto de Salud Carlos III (PI18/01662 to CR, co-funded with European FEDER contribution) and of the Programa de Actividades en Biomedicina de la Comunidad de Madrid-B2017/BMD-3671-INFLAMUNE. S.B.F is supported by Endocornea2, convenio colaboración CSIC, funded by Instituto de Investigación Fundación Jiménez Díaz. An institutional support of Fundación Ramón Areces to the CBMSO is also acknowledged. DGW and CCN are recipients of FPI fellowships from MINECO. NCA and AC are recipients of FPU fellowships from MECD. We also thank Dr. Phil Mason, who provided English language support, and Dr. Miguel A. Alonso, for helpful comments.
Author information
Authors and Affiliations
Contributions
DGW, NCA and JM contributed to the conception and experimental design. DGW, NCA, CCN and SBF performed the experiments and acquired the data. AC and CR help carry out in vivo experiments and isolated lung endothelial cells. DGW and NCA analyzed the data, IC and CR provided material support, and reviewed the manuscript, which was written by JM.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Colás-Algora, N., García-Weber, D., Cacho-Navas, C. et al. Compensatory increase of VE-cadherin expression through ETS1 regulates endothelial barrier function in response to TNFα. Cell. Mol. Life Sci. 77, 2125–2140 (2020). https://doi.org/10.1007/s00018-019-03260-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03260-9