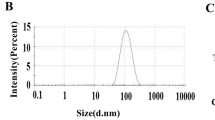
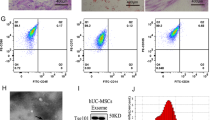
Abstract
Background and aims
Allogeneic human umbilical mesenchymal stem cells (alloUMSC) are convenient cell source for stem cell-based therapy. However, immune rejection is a major obstacle for clinical application of alloUMSC for cardiac repair after myocardial infarction (MI). The immune rejection is due to the presence of human leukocyte antigen (HLA) class I molecule which is increased during MI. The aim of this study was to knockout HLA light chain β2-microglobulin (B2M) in UMSC to enhance stem cell engraftment and survival after transplantation.
Methods and results
We developed an innovative strategy using CRISPR/Cas9 to generate UMSC with B2M deletion (B2M–UMSC). AlloUMSC injection induced CD8+ T cell-mediated immune rejection in immune competent rats, whereas no CD8+ T cell-mediated killing against B2M–UMSC was observed even when the cells were treated with IFN-γ. Moreover, we demonstrate that UMSC-derived exosomes can inhibit cardiac fibrosis and restore cardiac function, and exosomes derived from B2M–UMSC are more efficient than those derived from UMSC, indicating that the beneficial effect of exosomes can be enhanced by modulating exosome’s imprinting. Mechanistically, microRNA sequencing identifies miR-24 as a major component of the exosomes from B2M–UMSCs. Bioinformatics analysis identifies Bim as a putative target of miR-24. Loss-of-function studies at the cellular level and gain-of-function approaches in exosomes show that the beneficial effects of B2M–UMSCs are mediated by the exosome/miR-24/Bim pathway.
Conclusion
Our findings demonstrate that modulation of exosome’s imprinting via B2M knockout is an efficient strategy to prevent the immune rejection of alloUMSCs. This study paved the way to the development of new strategies for tissue repair and regeneration without the need for HLA matching.








Similar content being viewed by others
References
Karantalis V, Hare JM (2015) Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 116(8):1413–1430
Landin AM, Hare JM (2017) The quest for a successful cell-based therapeutic approach for heart failure. Eur Heart J 38(9):661–664
Huang XP et al (2010) Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 122(23):2419–2429
Riolobos L et al (2013) HLA engineering of human pluripotent stem cells. Mol Ther 21(6):1232–1241
Rubinstein P (2001) HLA matching for bone marrow transplantation–how much is enough? N Engl J Med 345(25):1842–1844
Wang D et al (2015) Targeted disruption of the beta2-microglobulin gene minimizes the immunogenicity of human embryonic stem cells. Stem Cells Transl Med 4(10):1234–1245
Tan K et al (2017) Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PLoS One 12(5):e0178624
Rink BE et al (2017) Isolation and characterization of equine endometrial mesenchymal stromal cells. Stem Cell Res Ther 8(1):166
Mandal PK et al (2014) Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15(5):643–652
Sahoo S, Losordo DW (2014) Exosomes and cardiac repair after myocardial infarction. Circ Res 114(2):333–344
Fang S et al (2016) Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl Med 5(10):1425–1439
Qian X et al (2016) Exosomal microRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis c virus infection. Stem Cells Transl Med 5(9):1190–1203
Cervio E et al (2015) Exosomes for intramyocardial intercellular communication. Stem Cells Int 2015:482171
Subra C et al (2010) Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res 51(8):2105–2120
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9(8):581–593
Teng X et al (2015) Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37(6):2415–2424
Boomsma RA, Geenen DL (2012) Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One 7(4):e35685
Lai RC et al (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4(3):214–222
Bian S et al (2014) Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92(4):387–397
Guo C et al (2015) Cardiomyocyte-specific role of miR-24 in promoting cell survival. J Cell Mol Med 19(1):103–112
Pan LJ et al (2017) MiR-24 alleviates cardiomyocyte apoptosis after myocardial infarction via targeting BIM. Eur Rev Med Pharmacol Sci 21(13):3088–3097
Hatzistergos KE et al (2010) Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 107(7):913–922
Kawada H et al (2004) Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 104(12):3581–3587
Padin-Iruegas ME et al (2009) Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation 120(10):876–887
Iso Y et al (2014) Priming with ligands secreted by human stromal progenitor cells promotes grafts of cardiac stem/progenitor cells after myocardial infarction. Stem Cells 32(3):674–683
Zentilin L et al (2010) Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J 24(5):1467–1478
Wang J et al (2012) MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med 16(9):2150–2160
Xiang Y et al (2015) Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood 125(22):3377–3387
Chevillet JR et al (2014) Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA 111(41):14888–14893
Lu P et al (2013) Generating hypoimmunogenic human embryonic stem cells by the disruption of beta 2-microglobulin. Stem Cell Rev 9(6):806–813
Deng Y et al (2016) Prostacyclin-producing human mesenchymal cells target H19 lncRNA to augment endogenous progenitor function in hindlimb ischaemia. Nat Commun 7:11276
Mathiyalagan P et al (2017) Angiogenic mechanisms of human CD34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res 120(9):1466–1476
Ammar HI et al (2015) Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res Ther 6:148
Mayourian J et al (2018) Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res 122(7):933–944
Hu Y et al (2018) Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 8(1):169–184
Qian L et al (2011) miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med 208(3):549–560
Maegdefessel L et al (2014) miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 5:5214
Coulson-Thomas VJ et al (2014) Umbilical cord mesenchymal stem cells suppress host rejection: the role of the glycocalyx. J Biol Chem 289(34):23465–23481
Huang WH et al (2014) Hypoxic mesenchymal stem cells engraft and ameliorate limb ischaemia in allogeneic recipients. Cardiovasc Res 101(2):266–276
de Almeida PE et al (2013) Immunogenicity of pluripotent stem cells and their derivatives. Circ Res 112(3):549–561
Swijnenburg RJ et al (2005) Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 112(9 Suppl):I166–I172
Swijnenburg RJ et al (2008) Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA 105(35):12991–12996
Singla DK (2016) Stem cells and exosomes in cardiac repair. Curr Opin Pharmacol 27:19–23
van Dongen HM et al (2016) Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol Mol Biol Rev 80(2):369–386
Li Y et al (2017) Dominant and recessive imprinting of exosomes from parent cells. Nat Rev Cardiol 14(8):491
Boon RA, Vickers KC (2013) Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol 33(2):186–192
Wolfers J et al (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7(3):297–303
Shahrivari M et al (2017) Peripheral blood cytokine levels after acute myocardial infarction: IL-1beta- and IL-6-related impairment of bone marrow function. Circ Res 120(12):1947–1957
Smith LK et al (2015) beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med 21(8):932–937
Sun Y et al (2018) miR-24 and miR-122 negatively regulate the transforming growth factor-beta/smad signaling pathway in skeletal muscle fibrosis. Mol Ther Nucleic Acids 11:528–537
Chen J et al (2013) mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res 112(12):1557–1566
Acknowledgement
We thank Dr. IC Bruce for English editing of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, No. 81870194, No. 91849122, No. 91839101), Jiangsu Province Key Scientific and Technological Project (BE2016669), Suzhou Science and Technology Project (SS201665), Jiangsu Province Peak of Talent in Six Industries (BU24600117), National Natural Science Foundation of China (No. U1601227 to X. Y. Y.), Science and Technology Programs of Guangdong Province (No. 2015B020225006 to X. Y. Y.).
Author information
Authors and Affiliations
Contributions
YL conceived, designed the study, analyzed data and wrote the manuscript. LS, YZ, YZ, YW, BY and WX performed the experiments and collected data. CL, BL, XP, YS, ZS and XY interpreted the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Research involving animal participants
The animal experiments were approved by the Animal Care and Use Committee of Soochow University.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lianbo Shao, Yu Zhang, Xiangbin Pan, Bin Liu and Chun Liang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, L., Zhang, Y., Pan, X. et al. Knockout of beta-2 microglobulin enhances cardiac repair by modulating exosome imprinting and inhibiting stem cell-induced immune rejection. Cell. Mol. Life Sci. 77, 937–952 (2020). https://doi.org/10.1007/s00018-019-03220-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03220-3