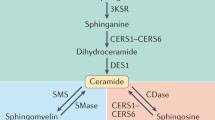
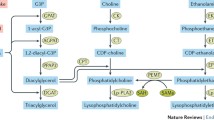
Abstract
Emerging evidence shows that palmitic acid (PA), a common fatty acid in the human diet, serves as a signaling molecule regulating the progression and development of many diseases at the molecular level. In this review, we focus on its regulatory roles in the development of five pathological conditions, namely, metabolic syndrome, cardiovascular diseases, cancer, neurodegenerative diseases, and inflammation. We summarize the clinical and epidemiological studies; and also the mechanistic studies which have identified the molecular targets for PA in these pathological conditions. Activation or inactivation of these molecular targets by PA controls disease development. Therefore, identifying the specific targets and signaling pathways that are regulated by PA can give us a better understanding of how these diseases develop for the design of effective targeted therapeutics.

Similar content being viewed by others
References
Gunstone FD, Harwood JL, Dijkstra AJ (2007) The lipid handbook with CD-ROM. CRC Press, Boca Raton
Bier DM (2016) Saturated fats and cardiovascular disease: interpretations not as simple as they once were. Crit Rev Food Sci Nutr 56:1943–1946
Mancini A et al (2015) Biological and nutritional properties of palm oil and palmitic acid: effects on health. Molecules (Basel, Switzerland) 20:17339–17361
Hermann JR (2017) Diet and heart disease Oklahoma cooperative extension service T3160. http://factsheets.okstate.edu/documents/t-3160-diet-and-heart-disease. Accessed 18 June 2018
Carta G et al (2017) Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol 8:902
Yu Y et al (2012) Serum levels of polyunsaturated fatty acids are low in Chinese men with metabolic syndrome, whereas serum levels of saturated fatty acids, zinc, and magnesium are high. Nutr Res (New York, N.Y.) 32:71–77
Klein S, Wolfe RR (1992) Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol 262:E631–E636
Abdelmagid SA et al (2015) Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One 10(2):e0116195
Gehrmann W et al (2010) Role of metabolically generated reactive oxygen species for lipotoxicity in pancreatic beta-cells. Diabetes Obes Metab 12(Suppl 2):149–158
Jensen MD et al (1989) Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 83:1168–1173
Abdelmagid SA et al (2015) Comprehensive profiling of plasma fatty acid concentration in young healthy Canadian adults. PLoS One 10(2):e0116195
El-Ansary Afaf K et al (2011) Plasma fatty acids as diagnostic markers in autistic patients from Saudi Arabia. Lipids Health Dis 10:62
Cunnane SC et al (2012) Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 29(3):691–697
Trombetta A et al (2013) Increase of palmitic acid concentration impairs endothelial progenitor cells and bone marrow-derived progenitor cell bioavailability. Diabetes 62:1245
Fraser DA et al (1999) Changes in plasma free fatty acid concentrations in rheumatoid arthritis patients during fasting and their effects upon T-lymphocyte proliferation. Rheumatology 38:948–952
Thomas GM, Huganir RL (2013) Palmitoylation-dependent regulation of glutamate receptors and their PDZ domain-containing partners. Biochem Soc Trans 41:72–78
Chamberlain LH et al (2013) Palmitoylation and the trafficking of peripheral membrane proteins. Biochem Soc Trans 41:62–66
Charest PG, Bouvier M (2003) Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem 278:41541–41551
Chen B et al (2018) protein lipidation in cell signaling and diseases: function, regulation, and therapeutic opportunities. Cell Chem Biol 25(7):817–831
Lin DTS, Davis NG, Conibear E (2017) Targeting the Ras palmitoylation/depalmitoylation cycle in cancer. Biochem Soc Trans 45:913–921
Adams MN et al (2011) The role of palmitoylation in signalling, cellular trafficking and plasma membrane localization of protease-activated receptor-2. PLoS One 6:e28018
Zhou B et al (2004) The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res 64:7455–7463
Resh MG (2017) Palmitoylation of proteins in cancer. Biochem Soc Trans 45:409–416
Anderson AM, Ragan MA (2016) Palmitoylation: a protein S-acylation with implications for breast cancer. NPJ Breast Cancer 2:16028
Sambataro F, Pennuto M (2017) Post-translational modifications and protein quality control in motor neuron and polyglutamine diseases. Front Mol Neurosci 10:82
Holland SM, Thomas GM (2017) Roles of palmitoylation in axon growth, degeneration and regeneration. J Neurosci Res 95:1528–1539
Cho E, Park M (2016) Palmitoylation in Alzheimer’s disease and other neurodegenerative diseases. Pharmacol Res 111:133–151
Mohammed AM, Chen F, Kowluru A (2013) The two faces of protein palmitoylation in islet beta-cell function: potential implications in the pathophysiology of islet metabolic dysregulation and diabetes. Recent Pat Endocr Metab Immune Drug Discov 7:203–212
Zhao L et al (2018) CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol 693:705–717
Paley CA, Johnson MI (2018) Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehab 10:7
Cooper-DeHoff RM, Pepine CJ (2007) Metabolic syndrome and cardiovascular disease: challenges and opportunities. Clin Cardiol 30:593–597
Kabagambe EK et al (2008) Erythrocyte fatty acid composition and the metabolic syndrome: a National Heart, Lung, and Blood Institute GOLDN study. Clin Chem 54:154–162
Cook SL et al (1997) Palmitic acid effect on lipoprotein profiles and endogenous cholesterol synthesis or clearance in humans. Asia Pacific J Clin Nutr 6:6–11
Kien CL, Bunn JY, Ugrasbul F (2005) Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr 82:320–326
Palomer X et al (2018) Palmitic and oleic acid: the yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endo Metab 29(3):178
Hoppa MB et al (2009) Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca2+ channels from secretory granules. Cell Metab 10:455–465
Cheng L et al (2015) Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J Biochem Nutr 26:541–548
Martino L et al (2012) Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS One 7:e36188
Graciano MF et al (2009) Palmitate activates insulin signaling pathway in pancreatic rat islets. Pancreas 38:578–584
Kennedy A et al (2009) Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr 139:1–4
Takahashi K et al (2008) JNK- and IkappaB-dependent pathways regulate MCP-1 but not adiponectin release from artificially hypertrophied 3T3-L1 adipocytes preloaded with palmitate in vitro. Am J Physiol Endocrinol Metab 294:E898–E909
Xi L et al (2007) Crocetin attenuates palmitate-induced insulin insensitivity and disordered tumor necrosis factor-alpha and adiponectin expression in rat adipocytes. Br J Pharmacol 151:610–617
Kim JY et al (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117:2621–2637
Bays HE et al (2008) Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardio Ther 6:343–368
Ajuwon KM, Spurlock ME (2005) Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr 135:1841–1846
Bradley RL, Fisher FF, Maratos-Flier E (2008) Dietary fatty acids differentially regulate production of TNF-alpha and IL-10 by murine 3T3-L1 adipocytes. Obesity (Silver Spring, Md.) 16:938–944
Youssef-Elabd EM et al (2012) Acute and chronic saturated fatty acid treatment as a key instigator of the TLR-mediated inflammatory response in human adipose tissue, in vitro. J Nutr Biochem 23:39–50
Guo W et al (2007) Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endo Metab 293:E576–E586
Jeon MJ et al (2012) Mitochondrial dysfunction and activation of iNOS are responsible for the palmitate-induced decrease in adiponectin synthesis in 3T3L1 adipocytes. Exp Mol Med 44:562–570
McCall KD et al (2010) Phenylmethimazole blocks palmitate-mediated induction of inflammatory cytokine pathways in 3T3L1 adipocytes and RAW 264.7 macrophages. J Endocrinol 207:343–353
Strissel KJ et al (2007) Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918
Cinti S et al (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355
Myers MG et al (2010) Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metabol 21:643–651
Crujeiras AB et al (2015) Leptin resistance in obesity: an epigenetic landscape. Life Sci 140:57–63
de Git KCG et al (2018) Is leptin resistance the cause or the consequence of diet-induced obesity? Int J Obesit 42(8):1445–1457
Benoit SC et al (2009) Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest 119(9):2577–2589
Qiao Q et al (2007) Metabolic syndrome and cardiovascular disease. Ann Clin Biochem 44:232–263
Wilson PW et al (2005) Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112:3066–3072
Dokken BB et al (2008) The Pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectr 21(3):160–165
Leon BM, Maddox TM (2015) Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 6(13):1246–1258
Mozaffarian D (2014) Saturated fatty acids and type 2 diabetes: more evidence to re-invent dietary guidelines. Lancet Diabetes Endocrinol 2(10):770–772
Ebbesson SO et al (2015) Fatty acids linked to cardiovascular mortality are associated with risk factors. Int J Circumpolar Health 74:28055
Briggs MA, Petersen KS, Kris-Etherton PM (2017) Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthcare 5(2):29
Knowles CJ et al (2013) Palmitate diet-induced loss of cardiac caveolin-3: a novel mechanism for lipid-induced contractile dysfunction. PLoS One 8:e61369
Mao Y et al (2017) STING-IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler Thromb Vasc Biol 37:920–929
Wang XL et al (2006) Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes 55:2301–2310
Yuan L et al (2017) Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. J Biol Chem 292:15002–15015
Trombetta A et al (2013) Increase of palmitic acid concentration impairs endothelial progenitor cell and bone marrow-derived progenitor cell bioavailability: role of the STAT5/PPARgamma transcriptional complex. Diabetes 62:1245–1257
Huang JV et al (2012) PPAR-gamma as a therapeutic target in cardiovascular disease: evidence and uncertainty. J Lipid Res 53:1738–1754
Kvandova M, Majzunova M, Dovinova I (2016) The role of PPARgamma in cardiovascular diseases. Physiol Res 65:S343–S363
Cheon HG, Cho YS (2014) Protection of palmitic acid-mediated lipotoxicity by arachidonic acid via channeling of palmitic acid into triglycerides in C2C12. J Biomed Sci 21:13
Sinha S et al (2004) Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279:41294–41301
Chen YP et al (2018) Acute hypoxic preconditioning prevents palmitic acid-induced cardiomyocyte apoptosis via switching metabolic GLUT4-glucose pathway back to CD36-fatty acid dependent. J Cell Biol 119:3363–3372
Bairwa SC, Parajuli N, Dyck JR (2016) The role of AMPK in cardiomyocyte health and survival. Biochimica Biophysica Acta 1862:2199–2210
Huang JP et al (2009) Impairment of insulin-stimulated Akt/GLUT4 signaling is associated with cardiac contractile dysfunction and aggravates I/R injury in STZ-diabetic rats. J Biochem Sci 16:77
Li J et al (2016) PKC-zeta interacts with STAT3 and promotes its activation in cardiomyocyte hypertrophy. J Pharma Sci 132:15–23
Kwan HY et al (2014) Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J Biol Chem 289:30525–30537
Wong RH et al (2009) A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell 136:1056–1072
Wang Y et al (2015) Transcriptional regulation of hepatic lipogenesis. Nature reviews. Mol Cell Biol 16:678–689
DeBerardinis RJ et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104:19345–19350
Kwan HY et al (2015) Dietary lipids and adipocytes: potential therapeutic targets in cancers. J Nutr Biochem 26:303–311
Little JL, Kridel SJ (2008) Fatty acid synthase activity in tumor cells. Subcell Biochem 49:169–194
Rohrig F, Schulze A (2016) The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer 16:732–749
Kwan HY et al (2013) The anticancer effect of oridonin is mediated by fatty acid synthase suppression in human colorectal cancer cells. J Gastroenterol 48:182–192
Ventura R et al (2015) Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine 2:808–824
Bensaad K et al (2014) Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep 9:349–365
Kuemmerle NB et al (2011) Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther 10:427–436
Laurent V et al (2016) Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun 7:10230
Dirat B et al (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71:2455–2465
Okumura T et al (2017) Extra-pancreatic invasion induces lipolytic and fibrotic changes in the adipose microenvironment, with released fatty acids enhancing the invasiveness of pancreatic cancer cells. Oncotarget 8:18280–18295
Wang YY et al (2017) Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2:e87489
Beloribi-Djefaflia S, Vasseur S, Guillaumond F (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5:e189
Louie SM et al (2013) Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochimica Biophysica Acta 1831:1566–1572
Benjamin DI, Cravatt BF, Nomura DK (2012) Global profiling strategies for mapping dysregulated metabolic pathways in cancer. Cell Metab 16:565–577
Pascual G et al (2017) Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541:41–45
Nath A et al (2015) Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Reps 5:14752
Balaban S et al (2017) Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 5:1
Binker-Cosen MJ et al (2017) Palmitic acid increases invasiveness of pancreatic cancer cells AsPC-1 through TLR4/ROS/NF-kappaB/MMP-9 signaling pathway. Biochem Biophys Res Commun 484:52–158
Lin L et al (2017) Functional lipidomics: palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology (Baltimore, Md.) 66:432–448
Baumann J et al (2016) Palmitate-induced ER stress increases trastuzumab sensitivity in HER2/neu-positive breast cancer cells. BMC Cancer 16:55
Fiorentino M et al (2008) verexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Investig Tech Methods Pathol 88:1340–1348
Sefton BM et al (1982) The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell 31:465–474
Buss JE, Sefton BM (1986) Direct identification of palmitic acid as the lipid attached to p21ras. Mol Cell Biol 6:116–122
Hancock JF et al (1989) All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57:1167–1177
Kato K, Der CJ, Buss JE (1992) Prenoids and palmitate: lipids that control the biological activity of Ras proteins. Semin Cancer Biol 3:179–188
Marwarha G et al (2017) Palmitate increases beta-site a betaPP-cleavage enzyme 1 activity and amyloid-beta genesis by evoking endoplasmic reticulum stress and subsequent C/EBP homologous protein activation. J Alzheimers Dis 57:907–925
Greenwood CE, Winocur G (2005) High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 26(Suppl 1):42–45
Baierle M et al (1973) Fatty acid status and its relationship to cognitive decline and homocysteine levels in the elderly. Nutrients 6(9):3624–3640
Dhopeshwarkar GA, Mead JF (1973) Uptake and transport of fatty acids into the brain and the role of the blood-brain barrier system. Adv Lipid Res 11:109–142
Liu L et al (2013) Palmitate induces transcriptional regulation of BACE1 and presenilin by STAT3 in neurons mediated by astrocytes. Exp Neurol 248:482–490
Patil S et al (2006) Palmitic acid-treated astrocytes induce BACE1 upregulation and accumulation of C-terminal fragment of APP in primary cortical neurons. Neurosci Lett 406:55–59
Patil S, Melrose J, Chan C (2007) Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur Neurosci 26:2131–2141
Wong KL et al (2014) Palmitic acid-induced lipotoxicity and protection by (+)-catechin in rat cortical astrocytes. Pharmacol Rep 66:1106–1113
Park HR et al (2011) Lipotoxicity of palmitic acid on neural progenitor cells and hippocampal neurogenesis. Toxicol Res 27:103–110
Ng YW, Say YH (2018) Palmitic acid induces neurotoxicity and gliatoxicity in SH-SY5Y human neuroblastoma and T98G human glioblastoma cells. Peer J 6:e4696
Conteduca V et al (2018) Association among metabolic syndrome, inflammation, and survival in prostate cancer. Urol Oncol 36:240.e241-240
Lopez-Candales A et al (2017) Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci 3(4):pii:e341
Wen H et al (2011) Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12:408–415
Shi H et al (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. The J Clin Invest 116:3015–3025
Nguyen MT et al (2007) A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282:35279–35292
Laine PS et al (2007) Palmitic acid induces IP-10 expression in human macrophages via NF-kappaB activation. Biochem Biophys Res Commun 358:150–155
Suganami T et al (2007) Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 27:84–91
Suganami T et al (2007) Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun 354:45–49
Song MJ et al (2006) Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 346:739–745
Gupta S et al (2012) Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Nutr Biochem 120:1060–1071
Tian D et al (2012) Overexpression of steroidogenic acute regulatory protein in rat aortic endothelial cells attenuates palmitic acid-induced inflammation and reduction in nitric oxide bioavailability. Cardiovas Diabetol 11:144
Li W et al (2016) EGFR inhibition blocks palmitic acid-induced inflammation in cardiomyocytes and prevents hyperlipidemia-induced cardiac injury in mice. Sci Rep 6:24580
Murumalla RK et al (2012) Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis 11:175
Wang Y et al (2017) Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Comm 8:13997
Acknowledgements
We thank for Dr Martha for her professional editing of the manuscript.
Funding
This work was partially supported by the Research Grant Council of HKSAR HKBU-22103017-ECS, Natural Science Foundation of Guangdong Province #2018A0303130122, and the Hong Kong Baptist University Grant FRG2/16-17/010 and FRG2/17-18/002.
Author information
Authors and Affiliations
Contributions
Conception and design: HYK and ZXB. Acquisition of information: SF, HYK, XJH, RHG, CHH, MTC, and HLXW. Writing and review of the manuscript: SF, HYK, and ZXB. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatima, S., Hu, X., Gong, RH. et al. Palmitic acid is an intracellular signaling molecule involved in disease development. Cell. Mol. Life Sci. 76, 2547–2557 (2019). https://doi.org/10.1007/s00018-019-03092-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03092-7