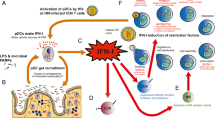
Abstract
Viral infections, including HIV, trigger the production of type I interferons (IFNs), which in turn, activate a signalling cascade that ultimately culminates with the expression of anti-viral proteins. Mounting evidence suggests that type I IFNs, in particular IFN-α, play a pivotal role in limiting acute HIV infection. Highly active anti-retroviral treatment reduces viral load and increases life expectancy in HIV positive patients; however, it fails to fully eliminate latent HIV reservoirs. To revisit HIV as a curable disease, this article reviews a body of literature that highlights type I IFNs as mediators in the control of HIV infection, with particular focus on the anti-HIV restriction factors induced and/or activated by IFN-α. In addition, we discuss the relevance of type I IFN treatment in the context of HIV latency reversal, novel therapeutic intervention strategies and the potential for full HIV clearance.


Similar content being viewed by others
References
Schoggins JW, Rice CM (2011) Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol 1:519–525
Hertzog PJ, Bourke NM, de Weerd NA, Mangan NE (2016) New interferons A2. In: Ratcliffe MJH (ed) Encyclopedia of immunobiology. Academic Press, Oxford, pp 501–508
Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA et al (2013) Interferon-α is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One 8:e56527
Cha L, Berry CM, Nolan D, Castley A, Fernandez S, French MA (2014) Interferon-alpha, immune activation and immune dysfunction in treated HIV infection. Clin Transl Immunol 3:e10
Gürtler C, Bowie AG (2013) Innate immune detection of microbial nucleic acids. Trends Microbiol 21:413–420
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N (2005) Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J Clin Investig 115:3265–3275
Lo CC, Schwartz JA, Johnson DJ, Yu M, Aidarus N, Mujib S, Benko E, Hyrcza M, Kovacs C, Ostrowski MA (2012) HIV delays IFN-α production from human plasmacytoid dendritic cells and is associated with SYK phosphorylation. PLoS One 7:e37052
Nascimbeni M, Perié L, Chorro L, Diocou S, Kreitmann L, Louis S, Garderet L, Fabiani B, Berger A, Schmitz J et al (2009) Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood 113:6112–6119
Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Tengchuan J, Laustsen A, Hansen K, Ostergaard L, Fitzgerald KA et al (2013) IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci USA 110:E4571–E4580
Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC (2014) IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343:428–432
Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J (2010) The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol 11:1005–1013
Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ (2013) Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341:903–906
de Weerd NA, Vivian JP, Nguyen TK, Mangan NE, Gould JA, Braniff SJ, Zaker-Tabrizi L, Fung KY, Forster SC, Beddoe T et al (2013) Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat Immunol 14:901–907
de Weerd NA, Samarajiwa SA, Hertzog PJ (2007) Type I interferon receptors: biochemistry and biological functions. J Biol Chem 282:20053–20057
Pestka S, Krause CD, Walter MR (2004) Interferons, interferon-like cytokines, and their receptors. Immunol Rev 202:8–32
Schindler C, Plumlee C (2008) Inteferons pen the JAK-STAT pathway. Semin Cell Dev Biol 19:311–318
Schindler C, Levy DE, Decker T (2007) JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282:20059–20063
Sadler AJ, Williams BRG (2008) Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559–568
Barré-Sinoussi F, Ross AL, Delfraissy JF (2013) Past, present and future: 30 years of HIV research. Nat Rev Microbiol 11:877–883
Doyle T, Goujon C, Malim MH (2015) HIV-1 and interferons: who’s interfering with whom? Nat Rev Microbiol 13:403–413
Kane M, Zang TM, Rihn SJ, Zhang F, Kueck T, Alim M, Schoggins J, Rice CM, Wilson SJ, Bieniasz PD (2016) Identification of interferon-stimulated genes with antiretroviral activity. Cell Host Microbe 20:392–405
Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J (2004) The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853
Danielson CM, Cianci GC, Hope TJ (2012) Recruitment and dynamics of proteasome association with rhTRIM5alpha cytoplasmic complexes during HIV-1 infection. Traffic 13:1206–1217
Rold CJ, Aiken C (2008) Proteasomal degradation of TRIM5alpha during retrovirus restriction. PLoS Pathog 4:e1000074
Liu FL, Qiu YQ, Li H, Kuang YQ, Tang X, Cao G, Tang NL, Zheng YT (2011) An HIV-1 resistance polymorphism in TRIM5alpha gene among Chinese intravenous drug users. J Acquir Immune Defic Syndr 56:306–311
Price H, Lacap P, Tuff J, Wachihi C, Kimani J, Ball TB, Luo M, Plummer FA (2010) A TRIM5alpha exon 2 polymorphism is associated with protection from HIV-1 infection in the Pumwani sex worker cohort. AIDS 24:1813–1821
Sewram S, Singh R, Kormuth E, Werner L, Mlisana K, Karim SS, Ndung’u T, Team CAIS (2009) Human TRIM5alpha expression levels and reduced susceptibility to HIV-1 infection. J Infect Dis 199:1657–1663
van Manen D, Rits MA, Beugeling C, van Dort K, Schuitemaker H, Kootstra NA (2008) The effect of Trim5 polymorphisms on the clinical course of HIV-1 infection. PLoS Pathog 4:e18
Ribeiro CM, Sarrami-Forooshani R, Setiawan LC, Zijlstra-Willems EM, van Hamme JL, Tigchelaar W, van der Wel NN, Kootstra NA, Gringhuis SI, Geijtenbeek TB (2016) Receptor usage dictates HIV-1 restriction by human TRIM5α in dendritic cell subsets. Nature 540:448–452
Sheehy AM, Gaddis NC, Malim MH (2003) The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med 9:1404–1407
Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM (2004) Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol 78:6073–6076
Goila-Gaur R, Strebel K (2008) HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology 5:51
Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH (2008) APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog 4:e1000231
Doehle BP, Schäfer A, Cullen BR (2005) Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology 339:281–288
Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW et al (2011) HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382
Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T et al (2012) SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13:223–228
Reinhard C, Bottinelli D, Kim B, Luban J (2014) Vpx rescue of HIV-1 from the antiviral state in mature dendritic cells is independent of the intracellular deoxynucleotide concentration. Retrovirology 11:12
Cribier A, Descours B, Valadão AL, Laguette N, Benkirane M (2013) Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 3:1036–1043
Sze A, Olagnier D, Lin R, van Grevenynghe J, Hiscott J (2013) SAMHD1 host restriction factor: a link with innate immune sensing of retrovirus infection. J Mol Biol 425:4981–4994
Goujon C, Moncorgé O, Bauby H, Doyle T, Ward CC, Schaller T, Hué S, Barclay WS, Schulz R, Malim MH (2013) Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502:559–562
Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, Schoggins JW, Rice CM, Yamashita M, Hatziioannou T, Bieniasz PD (2013) MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502:563–566
Liu Z, Pan Q, Ding S, Qian J, Xu F, Zhou J, Cen S, Guo F, Liang C (2013) The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14:398–410
Dicks MD, Goujon C, Pollpeter D, Betancor G, Apolonia L, Bergeron JR, Malim MH (2015) Oligomerization requirements for MX2-mediated suppression of HIV-1 infection. J Virol 90:22–32
Mavrommatis E, Fish EN, Platanias LC (2013) The schlafen family of proteins and their regulation by interferons. J Interferon Cytokine Res 33:206–210
Jakobsen MR, Mogensen TH, Paludan SR (2013) Caught in translation: innate restriction of HIV mRNA translation by a schlafen family protein. Cell Res 23:320–322
Li M, Kao E, Gao X, Sandig H, Limmer K, Pavon-Eternod M, Jones TE, Landry S, Pan T, Weitzman MD, David M (2012) Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature 491:125–128
Douglas JL, Gustin JK, Viswanathan K, Mansouri M, Moses AV, Fruh K (2010) The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog 6:e1000913
Miyagi E, Andrew AJ, Kao S, Strebel K (2009) Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc Natl Acad Sci USA 106:2868–2873
Neil SJ, Zang T, Bieniasz PD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430
Sauter D (2014) Counteraction of the multifunctional restriction factor tetherin. Front Microbiol 5:163
Lu J, Pan Q, Rong L, He W, Liu SL, Liang C (2011) The IFITM proteins inhibit HIV-1 infection. J Virol 85:2126–2137
Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM (2011) A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485
Everitt AR, Clare S, Pertel T, John SP, Wash RS, Smith SE, Chin CR, Feeley EM, Sims JS, Adams DJ et al (2012) IFITM3 restricts the morbidity and mortality associated with influenza. Nature 484:519–523
Compton AA, Bruel T, Porrot F, Mallet A, Sachse M, Euvrard M, Liang C, Casartelli N, Schwartz O (2014) IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe 16:736–747
Tartour K, Appourchaux R, Gaillard J, Nguyen XN, Durand S, Turpin J, Beaumont E, Roch E, Berger G, Mahieux R et al (2014) IFITM proteins are incorporated onto HIV-1 virion particles and negatively imprint their infectivity. Retrovirology 11:103
Yu J, Li M, Wilkins J, Ding S, Swartz TH, Esposito AM, Zheng YM, Freed EO, Liang C, Chen BK, Liu SL (2015) IFITM proteins restrict HIV-1 infection by antagonizing the envelope glycoprotein. Cell Rep 13:145–156
Foster TL, Wilson H, Iyer SS, Coss K, Doores K, Smith S, Kellam P, Finzi A, Borrow P, Hahn BH, Neil SJ (2016) Resistance of transmitted founder HIV-1 to IFITM-mediated restriction. Cell Host Microbe 20:429–442
Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D et al (2009) Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83:3719–3733
von Sydow M, Sönnerborg A, Gaines H, Strannegård O (1991) Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses 7:375–380
Cheney KM, McKnight Á (2010) Interferon-alpha mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication. PLoS One 5:e13521
Gendelman HE, Baca LM, Turpin J, Kalter DC, Hansen B, Orenstein JM, Dieffenbach CW, Friedman RM, Meltzer MS (1990) Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol 145:2669–2676
Goujon C, Malim MH (2010) Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol 84:9254–9266
Pitha PM (1994) Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antiviral Res 24:205–219
Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ et al (2014) Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–605
Cheng L, Yu H, Li G, Li F, Ma J, Li J, Chi L, Zhang L, Su L (2017) Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight 2(12):e94366
Zhen A, Rezek V, Youn C, Lam B, Chang N, Rick J, Carrillo M, Martin H, Kasparian S, Syed P et al (2017) Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J Clin Investig 127:260–268
Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH (2008) Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci USA 105:7034–7039
Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F et al (2013) Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 10:146
Rasaiyaah J, Tan CP, Fletcher AJ, Price AJ, Blondeau C, Hilditch L, Jacques DA, Selwood DL, James LC, Noursadeghi M, Towers GJ (2013) HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 503:402–405
Stevenson NJ, Bourke NM, Ryan EJ, Binder M, Fanning L, Johnston JA, Hegarty JE, Long A, O’Farrelly C (2013) Hepatitis C virus targets the interferon-α JAK/STAT pathway by promoting proteasomal degradation in immune cells and hepatocytes. FEBS Lett 587:1571–1578
Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, Buick R, Stevenson NJ, Touzelet O, Gadina M et al (2007) Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol 81:3428–3436
Bergeron JR, Huthoff H, Veselkov DA, Beavil RL, Simpson PJ, Matthews SJ, Malim MH, Sanderson MR (2010) The SOCS-box of HIV-1 Vif interacts with ElonginBC by induced-folding to recruit its Cul5-containing ubiquitin ligase complex. PLoS Pathog 6:e1000925
Sheehy AM, Gaddis NC, Choi JD, Malim MH (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646–650
Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J (2008) The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252
Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V (2009) HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog 5:e1000574
Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK (2014) HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046
Arias JF, Colomer-Lluch M, von Bredow B, Greene JM, MacDonald J, O’Connor DH, Serra-Moreno R, Evans DT (2016) Tetherin antagonism by HIV-1 group M Nef proteins. J Virol 90:10701–10714
Kluge SF, Mack K, Iyer SS, Pujol FM, Heigele A, Learn GH, Usmani SM, Sauter D, Joas S, Hotter D et al (2014) Nef proteins of epidemic HIV-1 group O strains antagonize human tetherin. Cell Host Microbe 16:639–650
Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J (2011) Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661
Luxon BA, Grace M, Brassard D, Bordens R (2002) Pegylated interferons for the treatment of chronic hepatitis C infection. Clin Ther 24:1363–1383
Kovacs JA, Deyton L, Davey R, Falloon J, Zunich K, Lee D, Metcalf JA, Bigley JW, Sawyer LA, Zoon KC (1989) Combined zidovudine and interferon-alpha therapy in patients with Kaposi sarcoma and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 111:280–287
Krown SE, Real FX, Cunningham-Rundles S, Myskowski PL, Koziner B, Fein S, Mittelman A, Oettgen HF, Safai B (1983) Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi’s sarcoma. N Engl J Med 308:1071–1076
Shepherd FA, Beaulieu R, Gelmon K, Thuot CA, Sawka C, Read S, Singer J (1998) Prospective randomized trial of two dose levels of interferon alfa with zidovudine for the treatment of Kaposi’s sarcoma associated with human immunodeficiency virus infection: a Canadian HIV Clinical Trials Network study. J Clin Oncol 16:1736–1742
Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, Chan ES, Schooley RT, Rinaldo CR, Thielman N et al (2010) Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J Infect Dis 201:1686–1696
Emilie D, Burgard M, Lascoux-Combe C, Laughlin M, Krzysiek R, Pignon C, Rudent A, Molina JM, Livrozet JM, Souala F et al (2001) Early control of HIV replication in primary HIV-1 infection treated with antiretroviral drugs and pegylated IFN alpha: results from the Primoferon A (ANRS 086) Study. AIDS 15:1435–1437
Utay NS, Douek DC (2016) Interferons and HIV infection: the good, the bad, and the ugly. Pathog Immun 1:107–116
Ambrus JL, Bardos TJ, Dembinski W, Chadha KC (2004) New approaches to the treatment of AIDS with special reference to overcoming interferon resistance. J Med 35:201–209
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R et al (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300
Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS (1997) Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA 94:13193–13197
Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF (2014) Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci USA 111:13475–13480
Pinkevych M, Cromer D, Tolstrup M, Grimm AJ, Cooper DA, Lewin SR, Søgaard OS, Rasmussen TA, Kent SJ, Kelleher AD, Davenport MP (2015) HIV reactivation from latency after treatment interruption occurs on average every 5–8 days-implications for HIV remission. PLoS Pathog 11:e1005000
Margolis DM, Garcia JV, Hazuda DJ, Haynes BF (2016) Latency reversal and viral clearance to cure HIV-1. Science 353:6517
Rasmussen TA, Tolstrup M, Søgaard OS (2016) Reversal of latency as part of a cure for HIV-1. Trends Microbiol 24:90–97
Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP et al (2013) Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis 207:213–222
Sun H, Buzon MJ, Shaw A, Berg RK, Yu XG, Ferrando-Martinez S, Leal M, Ruiz-Mateos E, Lichterfeld M (2014) Hepatitis C therapy with interferon-α and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis 209:1315–1320
Li P, Kaiser P, Lampiris HW, Kim P, Yukl SA, Havlir DV, Greene WC, Wong JK (2016) Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat Med 22:807–811
Acknowledgements
Thanks to Ms. Orla Convery for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourke, N.M., Napoletano, S., Bannan, C. et al. Control of HIV infection by IFN-α: implications for latency and a cure. Cell. Mol. Life Sci. 75, 775–783 (2018). https://doi.org/10.1007/s00018-017-2652-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2652-4