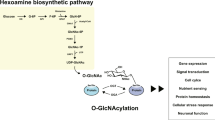
Abstract
O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) is involved in the regulation of many cellular cascades and neurological diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and stroke. In the brain, the expression of O-GlcNAcylation is notably heightened, as is that of O-linked N-acetylglucosaminyltransferase (OGT) and β-N-acetylglucosaminidase (OGA), the presence of which is prominent in many regions of neurological importance. Most importantly, O-GlcNAcylation is believed to contribute to the normal functioning of neurons; conversely, its dysregulation participates in the pathogenesis of neurological disorders. In neurodegenerative diseases, O-GlcNAcylation of the brain’s key proteins, such as tau and amyloid-β, interacts with their phosphorylation, thereby triggering the formation of neurofibrillary tangles and amyloid plaques. An increase of O-GlcNAcylation by pharmacological intervention prevents neuronal loss. Additionally, O-GlcNAcylation is stress sensitive, and its elevation is cytoprotective. Increased O-GlcNAcylation ameliorated brain damage in victims of both trauma-hemorrhage and stroke. In this review, we summarize the current understanding of O-GlcNAcylation’s physiological and pathological roles in the nervous system and provide a foundation for development of a therapeutic strategy for neurological disorders.





Similar content being viewed by others
Abbreviations
- Aβ:
-
β-Amyloid peptide
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- AICD:
-
Amyloid precursor protein intracellular domain
- APP:
-
Amyloid precursor protein
- Bad:
-
BCL2-associated agonist of cell death
- BAX:
-
BCL2 associated X
- BEMAD:
-
Michael addition with dithiothreitol
- CDK5:
-
Cyclin-dependent kinase 5
- CSF:
-
Cerebral spinal fluid
- eNOS:
-
Endothelial nitric oxide synthase
- ETD:
-
Electron-transfer dissociation
- GalT:
-
β1-4-Galactosyltransferase
- GFAT:
-
l-Glutamine:fructose-6-phosphate amidotransferase
- GlcN:
-
Glucosamine
- HAT:
-
Histone acetyltransferase
- HBP:
-
Hexosamine biosynthetic pathway
- HD:
-
Huntington’s disease
- ICH:
-
Intracerebral hemorrhage
- LC:
-
Liquid chromatography
- IKK:
-
Inhibitor of nuclear factor κB kinase
- IκB:
-
Inhibitor of nuclear factor κB
- I/R:
-
Ischemia/reperfusion
- MAP:
-
Microtubule-associated protein
- MGEA5:
-
Meningioma expressed antigen 5
- mOGT:
-
Mitochondrial OGT
- MS:
-
Mass spectrometry
- NButGT:
-
1,2-Dideoxy-2′-propyl-alpha-d-glucopyranoso-[2,1-d]-Delta 2′-thiazoline
- ncOGA:
-
Nuclear and cytoplasmic OGA
- ncOGT:
-
Nucleocytoplasmic OGT
- NFTs:
-
Neurofibrillary tangles
- NLS:
-
Nuclear localization signal
- NO:
-
Nitric oxide
- OGA:
-
β-N-Acetylglucosaminidase
- nNOS:
-
Neuronal nitric oxide synthase
- O-GlcNAcylation:
-
O-linked β-N-acetylglucosaminylation
- OGT:
-
O-linked N-acetylglucosaminyltransferase
- PD:
-
Parkinson’s disease
- PKAcs:
-
Protein kinase A catalytic subunit
- PFK1:
-
Phosphofructokinase 1
- PHFs:
-
Paired helical filaments
- PUGNAc:
-
O-(2-Acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate
- RPLC:
-
Reversed-phase liquid chromatography
- sAPPβ:
-
Soluble amyloid precursor protein β
- SOD1:
-
Superoxide dismutase 1
- sOGA:
-
Short-form OGA
- sOGT:
-
Short OGT
- Stat:
-
Signal transducer and activator of transcription
- TPR:
-
Tetratricopeptide
- YY1:
-
Yin Yang 1
References
Torres CR, Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259(5):3308–3317
Holt GD, Hart GW (1986) The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem 261(17):8049–8057
Haltiwanger RS, Holt GD, Hart GW (1990) Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem 265(5):2563–2568
Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446(7139):1017–1022. doi:10.1038/nature05815
Comer FI, Hart GW (2001) Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40(26):7845–7852
Groves JA, Lee A, Yildirir G, Zachara NE (2013) Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 18(5):535–558. doi:10.1007/s12192-013-0426-y
Harwood KR, Hanover JA (2014) Nutrient-driven O-GlcNAc cycling - think globally but act locally. J Cell Sci 127(Pt 9):1857–1867. doi:10.1242/jcs.113233
Vaidyanathan K, Durning S, Wells L (2014) Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology. Crit Rev Biochem Mol Biol 49(2):140–163. doi:10.3109/10409238.2014.884535
Hart GW, Greis KD, Dong LY, Blomberg MA, Chou TY, Jiang MS, Roquemore EP, Snow DM, Kreppel LK, Cole RN et al (1995) O-linked N-acetylglucosamine: the “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol 376:115–123
Hu P, Shimoji S, Hart GW (2010) Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett 584(12):2526–2538. doi:10.1016/j.febslet.2010.04.044
Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, Lefebvre T (2008) Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J 22(8):2901–2911. doi:10.1096/fj.07-102509
Sakabe K, Wang Z, Hart GW (2010) Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci USA 107(46):19915–19920. doi:10.1073/pnas.1009023107
Lu L, Fan D, Hu CW, Worth M, Ma ZX, Jiang J (2016) Distributive O-GlcNAcylation on the highly repetitive C-terminal domain of RNA polymerase II. Biochemistry 55(7):1149–1158. doi:10.1021/acs.biochem.5b01280
Marshall S, Bacote V, Traxinger RR (1991) Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266(8):4706–4712
Slawson C, Copeland RJ, Hart GW (2010) O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35(10):547–555. doi:10.1016/j.tibs.2010.04.005
Banerjee PS, Hart GW, Cho JW (2013) Chemical approaches to study O-GlcNAcylation. Chem Soc Rev 42(10):4345–4357. doi:10.1039/c2cs35412h
Martinez MR, Dias TB, Natov PS, Zachara NE (2017) Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem Soc Trans 45(1):237–249. doi:10.1042/bst20160153
Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279(29):30133–30142. doi:10.1074/jbc.M403773200
Nagel AK, Ball LE (2015) Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation. Adv Cancer Res 126:137–166. doi:10.1016/bs.acr.2014.12.003
Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW (2001) Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem 293(2):169–177. doi:10.1006/abio.2001.5132
Kamemura K, Hayes BK, Comer FI, Hart GW (2002) Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem 277(21):19229–19235. doi:10.1074/jbc.M201729200
Ludemann N, Clement A, Hans V, Leschik J, Behl C, Brandt R (2005) O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS). J Biol Chem 280(36):31648–31658. doi:10.1074/jbc.M504395200
Fujiki R, Hashiba W, Sekine H, Yokoyama A, Chikanishi T, Ito S, Imai Y, Kim J, He HH, Igarashi K, Kanno J, Ohtake F, Kitagawa H, Roeder RG, Brown M, Kato S (2011) GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature 480(7378):557–560. doi:10.1038/nature10656
Cameron A, Giacomozzi B, Joyce J, Gray A, Graham D, Ousson S, Neny M, Beher D, Carlson G, O’Moore J, Shearman M, Hering H (2013) Generation and characterization of a rabbit monoclonal antibody site-specific for tau O-GlcNAcylated at serine 400. FEBS Lett 587(22):3722–3728. doi:10.1016/j.febslet.2013.09.042
Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW (1987) Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 104(5):1157–1164
Snow CM, Senior A, Gerace L (1987) Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol 104(5):1143–1156
Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, Cantrell DA (2016) Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol 17(6):712–720. doi:10.1038/ni.3439
Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC (2004) Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA 101(36):13132–13137. doi:10.1073/pnas.0403471101
Zhao P, Viner R, Teo CF, Boons GJ, Horn D, Wells L (2011) Combining high-energy C-trap dissociation and electron transfer dissociation for protein O-GlcNAc modification site assignment. J Proteome Res 10(9):4088–4104. doi:10.1021/pr2002726
Myers SA, Panning B, Burlingame AL (2011) Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci USA 108(23):9490–9495. doi:10.1073/pnas.1019289108
Zaro BW, Yang YY, Hang HC, Pratt MR (2011) Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci USA 108(20):8146–8151. doi:10.1073/pnas.1102458108
Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW (2002) Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteom 1(10):791–804
Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC (2007) Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol 3(6):339–348. doi:10.1038/nchembio881
Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG 2nd, Shabanowitz J, Stanley P, Hart GW, Hunt DF, Yang F, Smith RD (2012) Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA 109(19):7280–7285. doi:10.1073/pnas.1200425109
Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, Burlingame AL (2012) Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteom 11(8):215–229. doi:10.1074/mcp.O112.018366
Maury JJ, Ng D, Bi X, Bardor M, Choo AB (2014) Multiple reaction monitoring mass spectrometry for the discovery and quantification of O-GlcNAc-modified proteins. Anal Chem 86(1):395–402. doi:10.1021/ac401821d
Wang X, Yuan ZF, Fan J, Karch KR, Ball LE, Denu JM, Garcia BA (2016) A novel quantitative mass spectrometry platform for determining protein O-GlcNAcylation dynamics. Mol Cell Proteom 15(7):2462–2475. doi:10.1074/mcp.O115.049627
Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE (2010) O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem 285(50):39096–39107. doi:10.1074/jbc.M110.131102
Lim KH, Chang HI (2006) O-linked N-acetylglucosamine suppresses thermal aggregation of Sp1. FEBS Lett 580(19):4645–4652. doi:10.1016/j.febslet.2006.07.040
Olivier-Van Stichelen S, Dehennaut V, Buzy A, Zachayus JL, Guinez C, Mir AM, El Yazidi-Belkoura I, Copin MC, Boureme D, Loyaux D, Ferrara P, Lefebvre T (2014) O-GlcNAcylation stabilizes beta-catenin through direct competition with phosphorylation at threonine 41. FASEB J 28(8):3325–3338. doi:10.1096/fj.13-243535
Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Investig 108(9):1341–1348. doi:10.1172/jci11235
Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R (2002) Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106(4):466–472
Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC (2012) Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science (New York, NY) 337(6097):975–980. doi:10.1126/science.1222278
Charoensuksai P, Kuhn P, Wang L, Sherer N, Xu W (2015) O-GlcNAcylation of co-activator-associated arginine methyltransferase 1 regulates its protein substrate specificity. Biochem J 466(3):587–599. doi:10.1042/bj20141072
Schindler C, Levy DE, Decker T (2007) JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282(28):20059–20063. doi:10.1074/jbc.R700016200
Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B (2004) The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem 279(5):3563–3572. doi:10.1074/jbc.M306449200
Gordon S, Akopyan G, Garban H, Bonavida B (2006) Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene 25(8):1125–1142. doi:10.1038/sj.onc.1209080
Hiromura M, Choi CH, Sabourin NA, Jones H, Bachvarov D, Usheva A (2003) YY1 is regulated by O-linked N-acetylglucosaminylation (O-glcNAcylation). J Biol Chem 278(16):14046–14052. doi:10.1074/jbc.M300789200
Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, Hanover JA (2006) Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA 103(32):11952–11957. doi:10.1073/pnas.0601931103
Wang Z, Gucek M, Hart GW (2008) Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci USA 105(37):13793–13798. doi:10.1073/pnas.0806216105
Slawson C, Hart GW (2011) O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 11(9):678–684. doi:10.1038/nrc3114
Love DC (2005) Hanover JA (2005) The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Science’s STKE 312:re13. doi:10.1126/stke.3122005re13
Kreppel LK, Blomberg MA, Hart GW (1997) Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 272(14):9308–9315
Gao Y, Wells L, Comer FI, Parker GJ, Hart GW (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem 276(13):9838–9845. doi:10.1074/jbc.M010420200
Nolte D, Muller U (2002) Human O-GlcNAc transferase (OGT): genomic structure, analysis of splice variants, fine mapping in Xq13.1. Mamm Genome 13(1):62–64
Lubas WA, Hanover JA (2000) Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J Biol Chem 275(15):10983–10988
Blatch GL, Lassle M (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays 21(11):932–939. doi:10.1002/(sici)1521-1878(199911)21:11<932:aid-bies5>3.0.co;2-n
Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E (2004) The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol 11(10):1001–1007. doi:10.1038/nsmb833
Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE (2006) Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology 16(6):551–563. doi:10.1093/glycob/cwj096
Cheung WD, Hart GW (2008) AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem 283(19):13009–13020. doi:10.1074/jbc.M801222200
Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM (2008) Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451(7181):964–969. doi:10.1038/nature06668
Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S (2011) Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469(7331):564–567. doi:10.1038/nature09638
Nemeth AH, Nolte D, Dunne E, Niemann S, Kostrzewa M, Peters U, Fraser E, Bochukova E, Butler R, Brown J, Cox RD, Levy ER, Ropers HH, Monaco AP, Muller U (1999) Refined linkage disequilibrium and physical mapping of the gene locus for X-linked dystonia-parkinsonism (DYT3). Genomics 60(3):320–329. doi:10.1006/geno.1999.5929
Kemppinen AK, Kaprio J, Palotie A, Saarela J (2011) Systematic review of genome-wide expression studies in multiple sclerosis. BMJ Open 1(1):e000053. doi:10.1136/bmjopen-2011-000053
Hewagama A, Gorelik G, Patel D, Liyanarachchi P, McCune WJ, Somers E, Gonzalez-Rivera T, Strickland F, Richardson B (2013) Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun 41:60–71. doi:10.1016/j.jaut.2012.12.006
Comtesse N, Maldener E, Meese E (2001) Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun 283(3):634–640. doi:10.1006/bbrc.2001.4815
Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW (2008) Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem 283(35):23557–23566. doi:10.1074/jbc.M804116200
Hart GW, West CM (2009) Nucleocytoplasmic Glycosylation. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, New York
Hart GW, Akimoto Y (2009) The O-GlcNAc modification. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, New York
Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE (2000) Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science (New York, NY) 290(5500):2302–2303. doi:10.1126/science.290.5500.2302
Van Tine BA, Patterson A, Kudlow JE (2003) Assignment of N-acetyl-d-glucosaminidase (Mgea5) to rat chromosome 1q5 by tyramide fluorescence in situ hybridization (T-FISH): synteny between rat, mouse and human with Insulin Degradation Enzyme (IDE). Cytogenet Genome Res 103(1–2):202b
Horsch M, Hoesch L, Vasella A, Rast DM (1991) N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-N-acetylglucosaminidase. Eur J Biochem 197(3):815–818
Dong DL, Hart GW (1994) Purification and characterization of an O-GlcNAc selective N-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J Biol Chem 269(30):19321–19330
Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ (2005) O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem 280(27):25313–25322. doi:10.1074/jbc.M413819200
Ji S, Park SY, Roth J, Kim HS, Cho JW (2012) O-GlcNAc modification of PPARgamma reduces its transcriptional activity. Biochem Biophys Res Commun 417(4):1158–1163. doi:10.1016/j.bbrc.2011.12.086
Mehdy A, Morelle W, Rosnoblet C, Legrand D, Lefebvre T, Duvet S, Foulquier F (2012) PUGNAc treatment leads to an unusual accumulation of free oligosaccharides in CHO cells. J Biochem 151(4):439–446. doi:10.1093/jb/mvs012
Shan X, Vocadlo DJ, Krieger C (2012) Reduced protein O-glycosylation in the nervous system of the mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett 516(2):296–301. doi:10.1016/j.neulet.2012.04.018
Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ (2008) A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4(8):483–490. doi:10.1038/nchembio.96
Andres-Bergos J, Tardio L, Larranaga-Vera A, Gomez R, Herrero-Beaumont G, Largo R (2012) The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem 287(40):33615–33628. doi:10.1074/jbc.M112.354241
Yu Y, Zhang L, Li X, Run X, Liang Z, Li Y, Liu Y, Lee MH, Grundke-Iqbal I, Iqbal K, Vocadlo DJ, Liu F, Gong CX (2012) Differential effects of an O-GlcNAcase inhibitor on tau phosphorylation. PLoS One 7(4):e35277. doi:10.1371/journal.pone.0035277
Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, Vocadlo DJ (2012) Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol 8(4):393–399. doi:10.1038/nchembio.797
Graham DL, Gray AJ, Joyce JA, Yu D, O’Moore J, Carlson GA, Shearman MS, Dellovade TL, Hering H (2014) Increased O-GlcNAcylation reduces pathological tau without affecting its normal phosphorylation in a mouse model of tauopathy. Neuropharmacology 79:307–313. doi:10.1016/j.neuropharm.2013.11.025
Taylor EW, Wang K, Nelson AR, Bredemann TM, Fraser KB, Clinton SM, Puckett R, Marchase RB, Chatham JC, McMahon LL (2014) O-GlcNAcylation of AMPA receptor GluA2 is associated with a novel form of long-term depression at hippocampal synapses. J Neurosci 34(1):10–21. doi:10.1523/jneurosci.4761-12.2014
Lubas WA, Frank DW, Krause M, Hanover JA (1997) O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 272(14):9316–9324
Okuyama R, Marshall S (2003) UDP-N-acetylglucosaminyl transferase (OGT) in brain tissue: temperature sensitivity and subcellular distribution of cytosolic and nuclear enzyme. J Neurochem 86(5):1271–1280
Gong CX, Liu F, Iqbal K (2016) O-GlcNAcylation: a regulator of tau pathology and neurodegeneration. Alzheimer’s Dementia. doi:10.1016/j.jalz.2016.02.011
Liu Y, Li X, Yu Y, Shi J, Liang Z, Run X, Li Y, Dai CL, Grundke-Iqbal I, Iqbal K, Liu F, Gong CX (2012) Developmental regulation of protein O-GlcNAcylation, O-GlcNAc transferase, and O-GlcNAcase in mammalian brain. PLoS One 7(8):e43724. doi:10.1371/journal.pone.0043724
Rex-Mathes M, Werner S, Strutas D, Griffith LS, Viebahn C, Thelen K, Schmitz B (2001) O-GlcNAc expression in developing and ageing mouse brain. Biochimie 83(7):583–590
Liu K, Paterson AJ, Zhang F, McAndrew J, Fukuchi K, Wyss JM, Peng L, Hu Y, Kudlow JE (2004) Accumulation of protein O-GlcNAc modification inhibits proteasomes in the brain and coincides with neuronal apoptosis in brain areas with high O-GlcNAc metabolism. J Neurochem 89(4):1044–1055. doi:10.1111/j.1471-4159.2004.02389.x
Yang YR, Song S, Hwang H, Jung JH, Kim SJ, Yoon S, Hur JH, Park JI, Lee C, Nam D, Seo YK, Kim JH, Rhim H, Suh PG (2017) Memory and synaptic plasticity are impaired by dysregulated hippocampal O-GlcNAcylation. Sci Rep 7:44921. doi:10.1038/srep44921
O’Donnell N, Zachara NE, Hart GW, Marth JD (2004) Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 24(4):1680–1690
Ning X, Tao T, Shen J, Ji Y, Xie L, Wang H, Liu N, Xu X, Sun C, Zhang D, Shen A, Ke K (2017) The O-GlcNAc modification of CDK5 involved in neuronal apoptosis following in vitro intracerebral hemorrhage. Cell Mol Neurobiol 37(3):527–536. doi:10.1007/s10571-016-0391-y
Kim SJ, Yoo WS, Choi M, Chung I, Yoo JM, Choi WS (2016) Increased O-GlcNAcylation of NF-kappaB enhances retinal ganglion cell death in streptozotocin-induced diabetic retinopathy. Curr Eye Res 41(2):249–257. doi:10.3109/02713683.2015.1006372
Wang P, Lazarus B, Forsythe M, Love D, Krause M, Hanover J (2012) O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc Natl Acad Sci USA 109(43):17669–17674. doi:10.1073/pnas.1205748109
Lagerlof O, Slocomb JE, Hong I, Aponte Y, Blackshaw S, Hart GW, Huganir RL (2016) The nutrient sensor OGT in PVN neurons regulates feeding. Science (New York, NY) 351(6279):1293–1296. doi:10.1126/science.aad5494
Lagerlof O, Hart GW, Huganir RL (2017) O-GlcNAc transferase regulates excitatory synapse maturity. Proc Natl Acad Sci USA 114(7):1684–1689. doi:10.1073/pnas.1621367114
Lagerlof O, Hart GW (2014) O-GlcNAcylation of neuronal proteins: roles in neuronal functions and in neurodegeneration. Adv Neurobiol 9:343–366. doi:10.1007/978-1-4939-1154-7_16
Wang AC, Jensen EH, Rexach JE, Vinters HV, Hsieh-Wilson LC (2016) Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc Natl Acad Sci USA 113(52):15120–15125. doi:10.1073/pnas.1606899113
As Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 12(4):459–509
Fukuyama H, Ogawa M, Yamauchi H, Yamaguchi S, Kimura J, Yonekura Y, Konishi J (1994) Altered cerebral energy metabolism in Alzheimer’s disease: a PET study. J Nucl Med 35(1):1–6
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A (2003) Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 30(8):1104–1113. doi:10.1007/s00259-003-1194-1
Hoyer S (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol 490(1–3):115–125. doi:10.1016/j.ejphar.2004.02.049
Cai H, Cong WN, Ji S, Rothman S, Maudsley S, Martin B (2012) Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr Alzheimer Res 9(1):5–17
Protas HD, Chen K, Langbaum JB, Fleisher AS, Alexander GE, Lee W, Bandy D, de Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM (2013) Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol 70(3):320–325. doi:10.1001/2013.jamaneurol.286
Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ (2007) Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68(7):501–508. doi:10.1212/01.wnl.0000244749.20056.d4
Burns CM, Chen K, Kaszniak AW, Lee W, Alexander GE, Bandy D, Fleisher AS, Caselli RJ, Reiman EM (2013) Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology 80(17):1557–1564. doi:10.1212/WNL.0b013e31828f17de
Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K (2004) Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63(4):658–663
Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX (2008) Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett 582(2):359–364. doi:10.1016/j.febslet.2007.12.035
de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2(6):1101–1113. doi:10.1177/193229680800200619
Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2009) Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J Neurochem 111(1):242–249. doi:10.1111/j.1471-4159.2009.06320.x
Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Tzourio C, Gin H, Barberger-Gateau P (2011) Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology 76(6):518–525. doi:10.1212/WNL.0b013e31820b7656
Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2011) Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225(1):54–62. doi:10.1002/path.2912
Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P (1998) Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm 105(4–5):423–438. doi:10.1007/s007020050068
Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C (2010) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 31(2):224–243. doi:10.1016/j.neurobiolaging.2008.04.002
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Investig 122(4):1316–1338. doi:10.1172/jci59903
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimer’s Dis 7(1):63–80
Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX (2009) Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain 132(Pt 7):1820–1832. doi:10.1093/brain/awp099
Guo T, Noble W, Hanger DP (2017) Roles of tau protein in health and disease. Acta Neuropathol. doi:10.1007/s00401-017-1707-9
Ksiezak-Reding H, Liu WK, Yen SH (1992) Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res 597(2):209–219
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259(8):5301–5305
Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, Iqbal K (1998) Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys 357(2):299–309. doi:10.1006/abbi.1998.0813
Cho JH, Johnson GV (2004) Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J Neurochem 88(2):349–358
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261(13):6084–6089
Nukina N, Kosik KS, Selkoe DJ (1987) Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci USA 84(10):3415–3419
Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98(12):6923–6928. doi:10.1073/pnas.121119298
Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM (2006) An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci USA 103(25):9673–9678. doi:10.1073/pnas.0602913103
Uno Y, Iwashita H, Tsukamoto T, Uchiyama N, Kawamoto T, Kori M, Nakanishi A (2009) Efficacy of a novel, orally active GSK-3 inhibitor 6-Methyl-N-[3-[[3-(1-methylethoxy)propyl]carbamoyl]-1H-pyrazol-4-yl]pyridine-3-carboxamide in tau transgenic mice. Brain Res 1296:148–163. doi:10.1016/j.brainres.2009.08.034
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science (New York, NY) 309(5733):476–481. doi:10.1126/science.1113694
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284(19):12845–12852. doi:10.1074/jbc.M808759200
Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW (1996) The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem 271(46):28741–28744
Yuzwa SA, Yadav AK, Skorobogatko Y, Clark T, Vosseller K, Vocadlo DJ (2011) Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids 40(3):857–868. doi:10.1007/s00726-010-0705-1
Wang Z, Udeshi ND, O’Malley M, Shabanowitz J, Hunt DF, Hart GW (2010) Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteom 9(1):153–160. doi:10.1074/mcp.M900268-MCP200
Morris M, Knudsen GM, Maeda S, Trinidad JC, Ioanoviciu A, Burlingame AL, Mucke L (2015) Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci 18(8):1183–1189. doi:10.1038/nn.4067
Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX (2004) O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA 101(29):10804–10809. doi:10.1073/pnas.0400348101
Li X, Lu F, Wang JZ, Gong CX (2006) Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J Neurosci 23(8):2078–2086. doi:10.1111/j.1460-9568.2006.04735.x
Gatta E, Lefebvre T, Gaetani S, dos Santos M, Marrocco J, Mir AM, Cassano T, Maccari S, Nicoletti F, Mairesse J (2016) Evidence for an imbalance between tau O-GlcNAcylation and phosphorylation in the hippocampus of a mouse model of Alzheimer’s disease. Pharmacol Res 105:186–197. doi:10.1016/j.phrs.2016.01.006
Lefebvre T, Ferreira S, Dupont-Wallois L, Bussiere T, Dupire MJ, Delacourte A, Michalski JC, Caillet-Boudin ML (2003) Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—a role in nuclear localization. Biochem Biophys Acta 1619(2):167–176
Ryu IH, Lee KY, Do SI (2016) Abeta-affected pathogenic induction of S-nitrosylation of OGT and identification of Cys-NO linkage triplet. Biochem Biophys Acta 1864(5):609–621. doi:10.1016/j.bbapap.2016.02.003
Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25(4):402–405. doi:10.1038/78078
Terwel D, Lasrado R, Snauwaert J, Vandeweert E, Van Haesendonck C, Borghgraef P, Van Leuven F (2005) Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem 280(5):3963–3973. doi:10.1074/jbc.M409876200
Yuzwa SA, Cheung AH, Okon M, McIntosh LP, Vocadlo DJ (2014) O-GlcNAc modification of tau directly inhibits its aggregation without perturbing the conformational properties of tau monomers. J Mol Biol 426(8):1736–1752. doi:10.1016/j.jmb.2014.01.004
Borghgraef P, Menuet C, Theunis C, Louis JV, Devijver H, Maurin H, Smet-Nocca C, Lippens G, Hilaire G, Gijsen H, Moechars D, Van Leuven F (2013) Increasing brain protein O-GlcNAc-ylation mitigates breathing defects and mortality of Tau. P301L mice. PLoS One 8(12):e84442. doi:10.1371/journal.pone.0084442
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82(12):4245–4249
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science (New York, NY) 256(5054):184–185
Hardy J (2017) The discovery of Alzheimer-causing mutations in the APP gene and the formulation of the “amyloid cascade hypothesis”. FEBS J 284(7):1040–1044. doi:10.1111/febs.14004
Griffith LS, Mathes M, Schmitz B (1995) Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J Neurosci Res 41(2):270–278. doi:10.1002/jnr.490410214
Zheng BW, Yang L, Dai XL, Jiang ZF, Huang HC (2016) Roles of O-GlcNAcylation on amyloid-beta precursor protein processing, tau phosphorylation, and hippocampal synapses dysfunction in Alzheimer’s disease. Neurol Res 38(2):177–186. doi:10.1080/01616412.2015.1133485
Jacobsen KT, Iverfeldt K (2011) O-GlcNAcylation increases non-amyloidogenic processing of the amyloid-beta precursor protein (APP). Biochem Biophys Res Commun 404(3):882–886. doi:10.1016/j.bbrc.2010.12.080
Chun YS, Park Y, Oh HG, Kim TW, Yang HO, Park MK, Chung S (2015) O-GlcNAcylation promotes non-amyloidogenic processing of amyloid-beta protein precursor via inhibition of endocytosis from the plasma membrane. J Alzheimer’s Dis 44(1):261–275. doi:10.3233/jad-140096
Kim C, Nam DW, Park SY, Song H, Hong HS, Boo JH, Jung ES, Kim Y, Baek JY, Kim KS, Cho JW, Mook-Jung I (2013) O-linked beta-N-acetylglucosaminidase inhibitor attenuates beta-amyloid plaque and rescues memory impairment. Neurobiol Aging 34(1):275–285. doi:10.1016/j.neurobiolaging.2012.03.001
Yuzwa SA, Shan X, Jones BA, Zhao G, Woodward ML, Li X, Zhu Y, McEachern EJ, Silverman MA, Watson NV, Gong CX, Vocadlo DJ (2014) Pharmacological inhibition of O-GlcNAcase (OGA) prevents cognitive decline and amyloid plaque formation in bigenic tau/APP mutant mice. Mol Neurodegeneration 9:42. doi:10.1186/1750-1326-9-42
Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, Zhang L, Dai CL, Gu JH, Gong CX, Iqbal K, Liu F (2016) O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell 15(3):455–464. doi:10.1111/acel.12449
Samaranch L, Lorenzo-Betancor O, Arbelo JM, Ferrer I, Lorenzo E, Irigoyen J, Pastor MA, Marrero C, Isla C, Herrera-Henriquez J, Pastor P (2010) PINK1-linked parkinsonism is associated with Lewy body pathology. Brain 133(Pt 4):1128–1142. doi:10.1093/brain/awq051
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601. doi:10.1002/mds.26424
Lubbe S, Morris HR (2014) Recent advances in Parkinson’s disease genetics. J Neurol 261(2):259–266. doi:10.1007/s00415-013-7003-2
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4(11):600–609. doi:10.1038/ncpneuro0924
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet (London, England) 364(9440):1167–1169. doi:10.1016/s0140-6736(04)17103-1
Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harbor Perspect Med 2(1):a008888. doi:10.1101/cshperspect.a008888
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (New York, NY) 276(5321):2045–2047
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108. doi:10.1038/ng0298-106
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) Alpha-synuclein locus triplication causes Parkinson’s disease. Science (New York, NY) 302(5646):841. doi:10.1126/science.1090278
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173. doi:10.1002/ana.10795
Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA 106(47):20051–20056. doi:10.1073/pnas.0908005106
Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209(5):975–986. doi:10.1084/jem.20112457
Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM, Klenerman D (2012) Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell 149(5):1048–1059. doi:10.1016/j.cell.2012.03.037
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4(2):160–164. doi:10.1038/ncb748
Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA (2008) Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem 283(24):16895–16905. doi:10.1074/jbc.M800747200
Chen L, Periquet M, Wang X, Negro A, McLean PJ, Hyman BT, Feany MB (2009) Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Investig 119(11):3257–3265. doi:10.1172/jci39088
Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA (2010) Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci 30(9):3184–3198. doi:10.1523/jneurosci.5922-09.2010
Hejjaoui M, Butterfield S, Fauvet B, Vercruysse F, Cui J, Dikiy I, Prudent M, Olschewski D, Zhang Y, Eliezer D, Lashuel HA (2012) Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: alpha-synuclein phosphorylation at tyrosine 125. J Am Chem Soc 134(11):5196–5210. doi:10.1021/ja210866j
Brahmachari S, Ge P, Lee SH, Kim D, Karuppagounder SS, Kumar M, Mao X, Shin JH, Lee Y, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM, Ko HS (2016) Activation of tyrosine kinase c-Abl contributes to alpha-synuclein-induced neurodegeneration. J Clin Investig 126(8):2970–2988. doi:10.1172/jci85456
Gomez-Tortosa E, Newell K, Irizarry MC, Sanders JL, Hyman BT (2000) alpha-Synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol 99(4):352–357
Alexopoulou Z, Lang J, Perrett RM, Elschami M, Hurry ME, Kim HT, Mazaraki D, Szabo A, Kessler BM, Goldberg AL, Ansorge O, Fulga TA, Tofaris GK (2016) Deubiquitinase Usp8 regulates alpha-synuclein clearance and modifies its toxicity in Lewy body disease. Proc Natl Acad Sci USA 113(32):E4688–E4697. doi:10.1073/pnas.1523597113
Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ (2001) Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science (New York, NY) 293(5528):263–269. doi:10.1126/science.1060627
Kim SJ, Sung JY, Um JW, Hattori N, Mizuno Y, Tanaka K, Paik SR, Kim J, Chung KC (2003) Parkin cleaves intracellular alpha-synuclein inclusions via the activation of calpain. J Biol Chem 278(43):41890–41899. doi:10.1074/jbc.M306017200
Marotta NP, Lin Y, Lewis YE, Ambroso MR, Zaro BW, Roth MT, Arnold DB, Langen R, Pratt MR (2015) O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson’s disease. Nat Chem 7(11):913–920. doi:10.1038/nchem.2361
Lewis YE, Galesic A, Levine PM, De Leon CA, Lamiri N, Brennan CK, Pratt MR (2017) O-GlcNAcylation of alpha-synuclein at serine 87 reduces aggregation without affecting membrane binding. ACS Chem Biol. doi:10.1021/acschembio.7b00113
Bork K, Kannicht C, Nohring S, Reutter W, Weidemann W, Hart GW, Horstkorte R (2008) N-propanoylmannosamine interferes with O-GlcNAc modification of the tyrosine 3-monooxygenase and stimulates dopamine secretion. J Neurosci Res 86(3):647–652. doi:10.1002/jnr.21526
Nettelbladt CG, Alibergovic A, Ljungqvist O (1996) Pre-stress carbohydrate solution prevents fatal outcome after hemorrhage in 24-hour food-deprived rats. Nutrition (Burbank, Los Angeles County, Calif) 12(10):696–699
Jarhult J (1973) Osmotic fluid transfer from tissue to blood during hemorrhagic hypotension. Acta Physiol Scand 89(2):213–226. doi:10.1111/j.1748-1716.1973.tb05514.x
Ware J, Ljungqvist O, Norberg KA, Nylander G (1982) Osmolar changes in haemorrhage: the effects of an altered nutritional status. Acta Chir Scand 148(8):641–646
Mizock BA (2001) Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab 15(4):533–551. doi:10.1053/beem.2001.0168
Dassanayaka S, Jones SP (2014) O-GlcNAc and the cardiovascular system. Pharmacol Ther 142(1):62–71. doi:10.1016/j.pharmthera.2013.11.005
Yang SL, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB (2006) Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock 25(6):600–607. doi:10.1097/01.shk.0000209563.07693.db
Not LG, Marchase RB, Fulop N, Brocks CA, Chatham JC (2007) Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats. Shock 28(3):345–352. doi:10.1097/shk.0b013e3180487ebb
Fulop N, Zhang Z, Marchase R, Chatham J (2007) Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 292(5):H2227–H2236. doi:10.1152/ajpheart.01091.2006
Zou L, Yang S, Hu S, Chaudry I, Marchase R, Chatham J (2007) The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock 27(4):402–408. doi:10.1097/01.shk.0000245031.31859.29
Laczy B, Marsh S, Brocks C, Wittmann I, Chatham J (2010) Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol 299(5):H1715–H1727. doi:10.1152/ajpheart.00337.2010
Lunde I, Aronsen J, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang L, Carlson C (2011) Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genom 44(2):162–172. doi:10.1152/physiolgenomics.00016.2011
Zou LY, Yang SL, Champattanachai V, Hu SH, Chaudry IH, Marchase RB, Chatham JC (2009) Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-kappa B signaling. Am J Physiol Heart Circ Physiol 296(2):H515–H523. doi:10.1152/ajpheart.01025.2008
Shi J, Gu JH, Dai CL, Gu J, Jin X, Sun J, Iqbal K, Liu F, Gong CX (2015) O-GlcNAcylation regulates ischemia-induced neuronal apoptosis through AKT signaling. Sci Rep 5:14500. doi:10.1038/srep14500
Liu S, Sheng H, Yu Z, Paschen W, Yang W (2016) O-linked β-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice: implications for impaired functional recovery from ischemic stress. J Cereb Blood Flow Metab 36(2):393–398. doi:10.1177/0271678x15608393
Hwang S, Shin J, Hwang J, Kim S, Shin J, Oh E, Oh S, Kim J, Lee J, Han I (2010) Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia 58(15):1881–1892. doi:10.1002/glia.21058
He Y, Ma X, Li D, Hao J (2016) Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-kappaB p65 signaling. J Cereb Blood Flow Metab. doi:10.1177/0271678x16679671
Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y (1996) Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab 16(2):186–194. doi:10.1097/00004647-199603000-00002
Yamashima T (2012) Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem 120(4):477–494. doi:10.1111/j.1471-4159.2011.07596.x
Chen R, Gong P, Tao T, Gao Y, Shen J, Yan Y, Duan C, Wang J, Liu X (2017) O-GlcNAc glycosylation of nNOS promotes neuronal apoptosis following glutamate excitotoxicity. Cell Mol Neurobiol. doi:10.1007/s10571-017-0477-1
Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, Geater C, Morozko E, Stocksdale J, Glatzer JC, Pham JT, Ahmed I, Peng Q, Wadhwa H, Pletnikova O, Troncoso JC, Duan W, Snyder SH, Ranum LP, Thompson LM, Lloyd TE, Ross CA, Rothstein JD (2017) Mutant Huntingtin disrupts the nuclear pore complex. Neuron 94(1):93–107. doi:10.1016/j.neuron.2017.03.023.e106
Katzenberger RJ, Chtarbanova S, Rimkus SA, Fischer JA, Kaur G, Seppala JM, Swanson LC, Zajac JE, Ganetzky B, Wassarman DA (2015) Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. eLife. doi:10.7554/eLife.04790
Hu JH, Chernoff K, Pelech S, Krieger C (2003) Protein kinase and protein phosphatase expression in the central nervous system of G93A mSOD over-expressing mice. J Neurochem 85(2):422–431
Hu JH, Zhang H, Wagey R, Krieger C, Pelech SL (2003) Protein kinase and protein phosphatase expression in amyotrophic lateral sclerosis spinal cord. J Neurochem 85(2):432–442
Honchar MP, Bunge MB, Agrawal HC (1982) In vivo phosphorylation of neurofilament proteins in the central nervous system of immature rat and rabbit. Neurochem Res 7(4):365–372
Dong DL, Xu ZS, Chevrier MR, Cotter RJ, Cleveland DW, Hart GW (1993) Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J Biol Chem 268(22):16679–16687
Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX (2008) Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J 22(1):138–145. doi:10.1096/fj.07-8309com
Hauser SL, Chan JR, Oksenberg JR (2013) Multiple sclerosis: prospects and promise. Ann Neurol 74(3):317–327. doi:10.1002/ana.24009
Baudoin L, Issad T (2014) O-GlcNAcylation and inflammation: a vast territory to explore. Front Endocrinol 5:235. doi:10.3389/fendo.2014.00235
Zhang D, Cai Y, Chen M, Gao L, Shen Y, Huang Z (2015) OGT-mediated O-GlcNAcylation promotes NF-kappaB activation and inflammation in acute pancreatitis. Inflamm Res 64(12):943–952. doi:10.1007/s00011-015-0877-y
Kim HB, Lee SW, Mun CH, Yoon JY, Pai J, Shin I, Park YB, Lee SK, Cho JW (2015) O-linked N-acetylglucosamine glycosylation of p65 aggravated the inflammation in both fibroblast-like synoviocytes stimulated by tumor necrosis factor-alpha and mice with collagen induced arthritis. Arthritis Res Ther 17:248. doi:10.1186/s13075-015-0762-7
Liu R, Ma X, Chen L, Yang Y, Zeng Y, Gao J, Jiang W, Zhang F, Li D, Han B, Han R, Qiu R, Huang W, Wang Y, Hao J (2017) MicroRNA-15b suppresses Th17 differentiation and is associated with pathogenesis of multiple sclerosis by targeting O-GlcNAc transferase. J Immunol 198(7):2626–2639. doi:10.4049/jimmunol.1601727
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81571600, 81322018 and 81273287 to J.W.H., and 81401361 to X.F.M.) and the Youth Top-notch Talent Support Program (to J.W.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, X., Li, H., He, Y. et al. The emerging link between O-GlcNAcylation and neurological disorders. Cell. Mol. Life Sci. 74, 3667–3686 (2017). https://doi.org/10.1007/s00018-017-2542-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2542-9