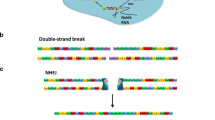
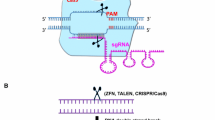
Abstract
Human pluripotent stem cells comprise induced pluripotent and embryonic stem cells, which have tremendous potential for biological and therapeutic applications. The development of efficient technologies for the targeted genome alteration of stem cells in disease models is a prerequisite for utilizing stem cells to their full potential. Genome editing of stem cells is possible with the help of synthetic nucleases that facilitate site-specific modification of a gene of interest. Recent advances in genome editing techniques have improved the efficiency and speed of the development of stem cells for human disease models. Zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated system are powerful tools for editing DNA at specific loci. Here, we discuss recent technological advances in genome editing with site-specific nucleases in human stem cells.


Similar content being viewed by others
References
Bibikova M (2003) Enhancing gene targeting with designed zinc finger nucleases. Science 300:764. doi:10.1126/science.1079512
Carroll D (2011) Genome engineering with zinc-finger nucleases. Genetics 188:773–782. doi:10.1534/genetics.111.131433
Capecchi MR (2001) Generating mice with targeted mutations. Nat Med 7:1086–1090
Smih F, Rouet P, Romanienko PJ, Jasin M (1995) Double-strand breaks at the target locus stimulate gene targeting in embryonic stem cells. Nucleic Acids Res 23:5012–5019
Choulika A, Perrin A, Dujon B, Nicolas JF (1995) Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol 15:1968–1973
Cohen-Tannoudji M, Robine S, Choulika A et al (1998) I-SceI-induced gene replacement at a natural locus in embryonic stem cells. Mol Cell Biol 18:1444–1448
Capecchi MR (1989) Altering the genome homologous recombination by from ES cells to germ line chimera. Science 244:1288–1292
Gloor G, Nassif N, Johnson-Schlitz D et al (1991) Targeted gene replacement in Drosophila via P element-induced gap repair. Science 253:1110–1117. doi:10.1126/science.1653452
Urnov FD, Rebar EJ, Holmes MC et al (2010) Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11:636–646. doi:10.1038/nrg2842
Wyman C, Kanaar R (2006) DNA double-strand break repair: All’s well that ends well. Annu Rev Genet 40:363–383. doi:10.1146/annurev.genet.40.110405.090451
Rouet P, Smih F, Jasin M (1994) Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci USA 91:6064–6068. doi:10.1073/pnas.91.13.6064
Gaj T, Gersbach CA, Barbas CF (2013) ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31:397–405. doi:10.1016/j.tibtech.2013.04.004
Lombardo A, Genovese P, Beausejour CM et al (2007) Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol 25:1298–1306. doi:10.1038/nbt1353
Mussolino C, Cathomen T (2012) TALE nucleases: tailored genome engineering made easy. Curr Opin Biotechnol 23:644–650. doi:10.1016/j.copbio.2012.01.013
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32:347–355. doi:10.1038/nbt.2842
Smith J, Grizot S, Arnould S et al (2006) A combinatorial approach to create artificial homing endonucleases cleaving chosen sequences. Nucleic Acids Res 34:e149. doi:10.1093/nar/gkl720
Boch J, Scholze H, Schornack S et al (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326:1509–1512. doi:10.1126/science.1178811
Ehrlich SD, Bolotin A, Quinquis B, Sorokin A (2005) Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151:2551–2561. doi:10.1099/mic.0.28048-0
Mojica FJM, Diez-Villasenor C, Garcia-Martinez J, Soria E (2005) Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 60:174–182. doi:10.1007/s00239-004-0046-3
Boettcher M, McManus MT (2015) Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol Cell 58:575–585. doi:10.1016/j.molcel.2015.04.028
Maeder ML, Thibodeau-beganny S, Osiak A et al (2008) Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Ther 31:294–301. doi:10.1016/j.molcel.2008.06.016.Rapid
Hiroyuki S, Susumu K (1981) New restriction endonucleases from Flavobacterium okeanokoites (FokI) and Micrococcus luteus (MluI). Gene 16:73–78. doi:10.1016/0378-1119(81)90062-7
Smith J, Berg JM, Chandrasegaran S (1999) A detailed study of the substrate specificity of a chimeric restriction enzyme. Nucleic Acids Res 27:674–681. doi:10.1093/nar/27.2.674
Chandrasegaran S, Smith J (1999) Chimeric restriction enzymes: what is next? Biol Chem. doi:10.1515/BC.1999.103
Urnov FD, Miller JC, Lee Y-L et al (2005) Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435:646–651. doi:10.1038/nature03556
Kandavelou K, Mani M, Durai S, Chandrasegaran S (2005) “Magic” scissors for genome surgery. Nat Biotechnol 23:686–687
Bogdanove AJ, Voytas DF (2011) TAL effectors: customizable proteins for DNA targeting. Science 333:1843–1846. doi:10.1126/science.1204094
Boch J, Bonas U (2010) Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol 48:419–436. doi:10.1146/annurev-phyto-080508-081936
Christian M, Cermak T, Doyle EL et al (2010) Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186:757–761. doi:10.1534/genetics.110.120717
Mahfouz MM, Li L, Shamimuzzaman M et al (2011) De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks. Proc Natl Acad Sci 108:2623–2628. doi:10.1073/pnas.1019533108
Li T, Huang S, Zhao X et al (2011) Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res 39:6315–6325. doi:10.1093/nar/gkr188
Mak AN-S, Bradley P, Cernadas RA et al (2012) The crystal structure of TAL effector PthXo1 bound to its DNA target. Science 335:716–719. doi:10.1126/science.1216211
Bultmann S, Morbitzer R, Schmidt CS et al (2012) Targeted transcriptional activation of silent oct4 pluripotency gene by combining designer TALEs and inhibition of epigenetic modifiers. Nucleic Acids Res 40:5368–5377. doi:10.1093/nar/gks199
Kim Y, Kweon J, Kim A et al (2013) A library of TAL effector nucleases spanning the human genome. Nat Biotechnol 31:251–258. doi:10.1038/nbt.2517
Valton J, Dupuy A, Daboussi F et al (2012) Overcoming transcription activator-like effector (TALE) DNA binding domain sensitivity to cytosine methylation. J Biol Chem 287:38427–38432. doi:10.1074/jbc.C112.408864
Deng D, Yin P, Yan C et al (2012) Recognition of methylated DNA by TAL effectors. Cell Res 22:1502–1504. doi:10.1038/cr.2012.127
Hockemeyer D, Wang H, Kiani S et al (2011) Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol 29:731–734. doi:10.1038/nbt.1927
Hockemeyer D, Soldner F, Beard C et al (2009) Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 27:851–857. doi:10.1038/nbt.1562
Moscou MJ, Bogdanove AJ (2009) A simple cipher governs DNA recognition by TAL effectors. Science 326:1501. doi:10.1126/science.1178817
Tesson L, Usal C, Ménoret S et al (2011) Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol 29:695–696. doi:10.1038/nbt.1940
Ding Q, Lee Y-K, Schaefer EAK et al (2013) A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 12:238–251. doi:10.1016/j.stem.2012.11.011
Holkers M, Maggio I, Liu J et al (2013) Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic Acids Res 41:e63. doi:10.1093/nar/gks1446
van der Oost J, Jore MM, Westra ER et al (2009) CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem Sci 34:401–407. doi:10.1016/j.tibs.2009.05.002
Jinek M, Chylinski K, Fonfara I et al (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. doi:10.1126/science.1225829
Haft DH, Selengut J, Mongodin EF, Nelson KE (2005) A Guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol 1:e60. doi:10.1371/journal.pcbi.0010060
Barrangou R, Fremaux C, Deveau H et al (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. doi:10.1126/science.1138140
Brouns SJJ, Jore MM, Lundgren M et al (2008) Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321:960–964. doi:10.1126/science.1159689
Deltcheva E, Chylinski K, Sharma CM et al (2011) CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471:602–607. doi:10.1038/nature09886
Cong L, Ran FA, Cox D et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. doi:10.1126/science.1231143
Mali P, Yang L, Esvelt KM et al (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826. doi:10.1126/science.1232033
Ran FA, Hsu PD, Wright J et al (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308
Nishimasu H, Ran FA, Hsu PD et al (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156:935–949. doi:10.1016/j.cell.2014.02.001
Swarts DC, Mosterd C, van Passel MWJ, Brouns SJJ (2012) CRISPR interference directs strand specific spacer acquisition. PLoS One 7:e35888. doi:10.1371/journal.pone.0035888
Kleinstiver BP, Prew MS, Tsai SQ et al (2015) Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523:481–485. doi:10.1038/nature14592
Kleinstiver BP, Pattanayak V, Prew MS et al (2016) High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529:490–495. doi:10.1038/nature16526
Thomson JA (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147. doi:10.1126/science.282.5391.1145
Urbach A (2004) Modeling for Lesch–Nyhan disease by gene targeting in human embryonic stem cells. Stem Cells 22:635–641. doi:10.1634/stemcells.22-4-635
Costa M, Dottori M, Sourris K et al (2007) A method for genetic modification of human embryonic stem cells using electroporation. Nat Protoc 2:792–796. doi:10.1038/nprot.2007.105
Di Domenico AI, Christodoulou I, Pells SC et al (2008) Sequential genetic modification of the hprt locus in human ESCs combining gene targeting and recombinase-mediated cassette exchange. Cloning Stem Cells 10:217–230. doi:10.1089/clo.2008.0016
Davis RP, Ng ES, Costa M et al (2008) Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 111:1876–1884. doi:10.1182/blood-2007-06-093609
Ruby KM, Zheng B (2009) Gene targeting in a HUES line of human embryonic stem cells via electroporation. Stem Cells 27:1496–1506. doi:10.1002/stem.73
Cowan CA, Klimanskaya I, McMahon J et al (2004) Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med 350:1353–1356. doi:10.1056/NEJMsr040330
Cartier N, Hacein-Bey-Abina S, Bartholomae CC et al (2009) Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326:818–823. doi:10.1126/science.1171242
Boudes PF (2014) Gene therapy as a new treatment option for inherited monogenic diseases. Eur J Intern Med 25:31–36. doi:10.1016/j.ejim.2013.09.009
Ibraheem D, Elaissari A, Fessi H (2014) Gene therapy and DNA delivery systems. Int J Pharm 459:70–83. doi:10.1016/j.ijpharm.2013.11.041
Hacein-Bey-Abina S, Le Deist F, Carlier F et al (2002) Sustained correction of X-Linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 346:1185–1193. doi:10.1056/NEJMoa012616
Hacein-Bey-Abina S (2003) LMO2-associated clonal T Cell proliferation in two patients after gene therapy for SCID-X1. Science 302:415–419. doi:10.1126/science.1088547
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4:346–358. doi:10.1038/nrg1066
Zou J, Maeder ML, Mali P et al (2009) Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell 5:97–110. doi:10.1016/j.stem.2009.05.023
Saleh-Gohari N (2004) Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res 32:3683–3688. doi:10.1093/nar/gkh703
Schwank G, Koo B-K, Sasselli V et al (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13:653–658. doi:10.1016/j.stem.2013.11.002
Suzuki K, Mitsui K, Aizawa E et al (2008) Highly efficient transient gene expression and gene targeting in primate embryonic stem cells with helper-dependent adenoviral vectors. Proc Natl Acad Sci 105:13781–13786. doi:10.1073/pnas.0806976105
Soldner F, Laganière J, Cheng AW et al (2011) Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146:318–331. doi:10.1016/j.cell.2011.06.019
Sebastiano V, Maeder ML, Angstman JF et al (2011) In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells 29:1717–1726. doi:10.1002/stem.718
Miller JC, Tan S, Qiao G et al (2011) A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29:143–148. doi:10.1038/nbt.1755
Park I-H, Zhao R, West JA et al (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146. doi:10.1038/nature06534
Raya Á, Rodríguez-Pizà I, Guenechea G et al (2009) Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460:53–59. doi:10.1038/nature08129
Dimos JT, Rodolfa KT, Niakan KK et al (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321:1218–1221. doi:10.1126/science.1158799
Ye Z, Zhan H, Mali P et al (2009) Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 114:5473–5480. doi:10.1182/blood-2009-04-217406
Jiang J, Jing Y, Cost GJ et al (2013) Translating dosage compensation to trisomy 21. Nature 500:296–300. doi:10.1038/nature12394
Brown CJ, Hendrich BD, Rupert JL et al (1992) The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71:527–542. doi:10.1016/0092-8674(92)90520-M
Kazuki Y, Yakura Y, Abe S et al (2014) Down syndrome-associated haematopoiesis abnormalities created by chromosome transfer and genome editing technologies. Sci Rep 4:6136. doi:10.1038/srep06136
Ramirez CL, Foley JE, Wright DA et al (2008) Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods 5:374–375. doi:10.1038/nmeth0508-374
Maeder ML, Thibodeau-Beganny S, Sander JD et al (2009) Oligomerized pool engineering (OPEN): an “open-source” protocol for making customized zinc-finger arrays. Nat Protoc 4:1471–1501. doi:10.1038/nprot.2009.98
Zhang F, Cong L, Lodato S et al (2011) Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol 29:149–153. doi:10.1038/nbt.1775
An MC, O’Brien RN, Zhang N et al (2014) Polyglutamine disease modeling: epitope based screen for homologous recombination using CRISPR/Cas9 system. PLoS Curr. doi:10.1371/currents.hd.0242d2e7ad72225efa72f6964589369a
Mandal PK, Ferreira LMR, Collins R et al (2014) Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15:643–652. doi:10.1016/j.stem.2014.10.004
Chen Y, Cao J, Xiong M et al (2015) Engineering human stem cell lines with inducible gene knockout using CRISPR/Cas9. Cell Stem Cell 17:233–244. doi:10.1016/j.stem.2015.06.001
Sapranauskas R, Gasiunas G, Fremaux C et al (2011) The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res 39:9275–9282. doi:10.1093/nar/gkr606
Davis L, Maizels N (2011) DNA nicks promote efficient and safe targeted gene correction. PLoS One 6:e23981. doi:10.1371/journal.pone.0023981
McConnell Smith A, Takeuchi R, Pellenz S et al (2009) Generation of a nicking enzyme that stimulates site-specific gene conversion from the I-AniI LAGLIDADG homing endonuclease. Proc Natl Acad Sci USA 106:5099–5104. doi:10.1073/pnas.0810588106
Metzger MJ, McConnell-Smith A, Stoddard BL, Miller AD (2011) Single-strand nicks induce homologous recombination with less toxicity than double-strand breaks using an AAV vector template. Nucleic Acids Res 39:926–935. doi:10.1093/nar/gkq826
Ran FA, Hsu PD, Lin C-Y et al (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389. doi:10.1016/j.cell.2013.08.021
Kim S, Kim D, Cho SW et al (2014) Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res 24:1012–1019. doi:10.1101/gr.171322.113
Cho SW, Kim S, Kim Y et al (2014) Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res 24:132–141. doi:10.1101/gr.162339.113
Fu Y, Sander JD, Reyon D et al (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284. doi:10.1038/nbt.2808
Wyvekens N, Topkar VV, Khayter C et al (2015) Dimeric CRISPR RNA-Guided FokI-dCas9 nucleases directed by truncated gRNAs for highly specific genome editing. Hum Gene Ther 26:425–431. doi:10.1089/hum.2015.084
Guilinger JP, Thompson DB, Liu DR (2014) Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32:577–582. doi:10.1038/nbt.2909
Tsai SQ, Wyvekens N, Khayter C et al (2014) Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32:569–576. doi:10.1038/nbt.2908
González F, Zhu Z, Shi Z-D et al (2014) An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell 15:215–226. doi:10.1016/j.stem.2014.05.018
Xie F, Ye L, Chang JC et al (2014) Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res 24:1526–1533. doi:10.1101/gr.173427.114
Zhang P-W, Haidet-Phillips AM, Pham JT et al (2016) Generation of GFAP:GFP astrocyte reporter lines from human adult fibroblast-derived iPS cells using zinc-finger nuclease technology. Glia 64:63–75. doi:10.1002/glia.22903
Tebas P, Stein D, Tang WW et al (2014) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370:901–910. doi:10.1056/NEJMoa1300662
Matano M, Date S, Shimokawa M et al (2015) Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. doi:10.1038/nm.3802
Drost J, van Jaarsveld RH, Ponsioen B et al (2015) Sequential cancer mutations in cultured human intestinal stem cells. Nature 521:43–47. doi:10.1038/nature14415
Martinez RA, Stein JL, Krostag A-RF et al (2015) Genome engineering of isogenic human ES cells to model autism disorders. Nucleic Acids Res 43:e65. doi:10.1093/nar/gkv164
Abudayyeh OO, Gootenberg JS, Konermann S et al (2016) C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353:aaf5573. doi:10.1126/science.aaf5573
Gao F, Shen XZ, Jiang F et al (2016) DNA-guided genome editing using the Natronobacterium gregoryi Argonaute. Nat Biotechnol 34:768–773. doi:10.1038/nbt.3547
Wang H (2016) NgAgo: an exciting new tool for genome editing. Sci Bull 61:1074–1075. doi:10.1007/s11434-016-1117-8
Lee SH, Turchiano G, Ata H et al (2016) Failure to detect DNA-guided genome editing using Natronobacterium gregoryi Argonaute. Nat Biotechnol. doi:10.1038/nbt.3753
Tiyaboonchai A, Mac H, Shamsedeen R et al (2014) Utilization of the AAVS1 safe harbor locus for hematopoietic specific transgene expression and gene knockdown in human ES cells. Stem Cell Res 12:630–637. doi:10.1016/j.scr.2014.02.004
Zou J, Sweeney CL, Chou B-K et al (2011) Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood 117:5561–5572. doi:10.1182/blood-2010-12-328161
Yusa K, Rashid ST, Strick-Marchand H et al (2011) Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells. Nature 478:391–394. doi:10.1038/nature10424
Wang J, Exline CM, DeClercq JJ et al (2015) Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors. Nat Biotechnol 33:1256–1263. doi:10.1038/nbt.3408
Asuri P, Bartel MA, Vazin T et al (2012) Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol Ther 20:329–338. doi:10.1038/mt.2011.255
Hofer U, Henley JE, Exline CM et al (2013) Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice. J Infect Dis 208:S160–S164. doi:10.1093/infdis/jit382
Li L, Krymskaya L, Wang J et al (2013) Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol Ther 21:1259–1269. doi:10.1038/mt.2013.65
Ma N, Liao B, Zhang H et al (2013) Transcription activator-like effector nuclease (TALEN)-mediated gene correction in integration-free β-thalassemia induced pluripotent stem cells. J Biol Chem 288:34671–34679. doi:10.1074/jbc.M113.496174
Osborn MJ, Starker CG, McElroy AN et al (2013) TALEN-based gene correction for Epidermolysis Bullosa. Mol Ther 21:1151–1159. doi:10.1038/mt.2013.56
Choi SM, Kim Y, Shim JS et al (2013) Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology 57:2458–2468. doi:10.1002/hep.26237
Park C-Y, Kim J, Kweon J et al (2014) Targeted inversion and reversion of the blood coagulation factor 8 gene in human iPS cells using TALENs. Proc Natl Acad Sci 111:9253–9258. doi:10.1073/pnas.1323941111
Cerbini T, Funahashi R, Luo Y et al (2015) Transcription activator-like effector nuclease (TALEN)-mediated CLYBL targeting enables enhanced transgene expression and one-step generation of dual reporter human induced pluripotent stem cell (iPSC) and neural stem cell (NSC) lines. PLoS One 10:e0116032. doi:10.1371/journal.pone.0116032
Mock U, Machowicz R, Hauber I et al (2015) mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res 43:5560–5571. doi:10.1093/nar/gkv469
Li HL, Fujimoto N, Sasakawa N et al (2015) Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Rep 4:143–154. doi:10.1016/j.stemcr.2014.10.013
Ye L, Wang J, Beyer AI et al (2014) Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Δ32 mutation confers resistance to HIV infection. Proc Natl Acad Sci USA 111:9591–9596. doi:10.1073/pnas.1407473111
Ding Q, Regan SN, Xia Y et al (2013) Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell 12:393–394. doi:10.1016/j.stem.2013.03.006
Huang X, Wang Y, Yan W et al (2015) Production of gene-corrected adult beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells 33:1470–1479. doi:10.1002/stem.1969
Bassuk AG, Zheng A, Li Y et al (2016) Precision medicine: genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci Rep 6:19969. doi:10.1038/srep19969
Firth AL, Menon T, Parker GS et al (2015) Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep 12:1385–1390. doi:10.1016/j.celrep.2015.07.062
Giani FC, Fiorini C, Wakabayashi A et al (2015) Targeted application of human genetic variation can improve red blood cell production from stem cells. Cell Stem Cell. doi:10.1016/j.stem.2015.09.015
Shalem O, Sanjana NE, Hartenian E et al (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343:84–87. doi:10.1126/science.1247005
Dever DP, Bak RO, Reinisch A et al (2016) CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature 539:384–389. doi:10.1038/nature20134
Acknowledgements
We would like to thank all of Suri’s laboratory members for their helpful discussions. Our work is supported by the National Research Foundation of Korea (2015R1C1A1A01054482 and 2013R1A1A1075992).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chandrasekaran, A.P., Song, M. & Ramakrishna, S. Genome editing: a robust technology for human stem cells. Cell. Mol. Life Sci. 74, 3335–3346 (2017). https://doi.org/10.1007/s00018-017-2522-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2522-0