Abstract
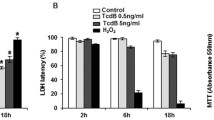
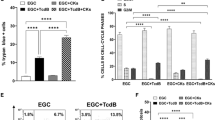
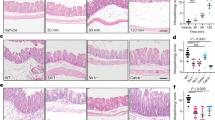
Clostridium difficile causes nosocomial/antibiotic-associated diarrhoea and pseudomembranous colitis. The major virulence factors are toxin A and toxin B (TcdB), which inactivate GTPases by monoglucosylation, leading to cytopathic (cytoskeleton alteration, cell rounding) and cytotoxic effects (cell-cycle arrest, apoptosis). C. difficile toxins breaching the intestinal epithelial barrier can act on underlying cells, enterocytes, colonocytes, and enteric neurons, as described in vitro and in vivo, but until now no data have been available on enteric glial cell (EGC) susceptibility. EGCs are crucial for regulating the enteric nervous system, gut homeostasis, the immune and inflammatory responses, and digestive and extradigestive diseases. Therefore, we evaluated the effects of C. difficile TcdB in EGCs. Rat-transformed EGCs were treated with TcdB at 0.1–10 ng/ml for 1.5–48 h, and several parameters were analysed. TcdB induces the following in EGCs: (1) early cell rounding with Rac1 glucosylation; (2) early G2/M cell-cycle arrest by cyclin B1/Cdc2 complex inactivation caused by p27 upregulation, the downregulation of cyclin B1 and Cdc2 phosphorylated at Thr161 and Tyr15; and (3) apoptosis by a caspase-dependent but mitochondria-independent pathway. Most importantly, the stimulation of EGCs with TNF-α plus IFN-γ before, concomitantly or after TcdB treatment strongly increased TcdB-induced apoptosis. Furthermore, EGCs that survived the cytotoxic effect of TcdB did not recover completely and showed not only persistent Rac1 glucosylation, cell-cycle arrest and low apoptosis but also increased production of glial cell-derived neurotrophic factor, suggesting self-rescuing mechanisms. In conclusion, the high susceptibility of EGCs to TcdB in vitro, the increased sensitivity to inflammatory cytokines related to apoptosis and the persistence of altered functions in surviving cells suggest an important in vivo role of EGCs in the pathogenesis of C. difficile infection.
















Similar content being viewed by others
Abbreviations
- TcdA:
-
Toxin A of Clostridium difficile
- TcdB:
-
Toxin B of Clostridium difficile
- EGCs:
-
Enteric glial cells
- ENS:
-
Enteric nervous system
- GDNF:
-
Glial cell-derived neurotrophic factor
- NGF:
-
Nerve growth factor
- TNF-α:
-
Tumour necrosis factor-alpha
- IFN-γ:
-
Interferon-gamma
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Foetal bovine serum
- PI:
-
Propidium iodide
- IL-1β :
-
Interleukin-1beta
- U:
-
Densitometric units
- CDKs:
-
Cyclin-dependent kinases
- Cdc2:
-
Cell division cycle 2
- GFAP:
-
Glial fibrillary acidic protein
- PARP:
-
Poly (ADP-ribose) polymerase
- BAF:
-
Boc-Asp(OMe)-fluoromethylketone
- Z-DEVD-FMK:
-
Z-Asp-Glu-Val-Asp-fluoromethylketone
- ROCK1:
-
Rho-associated coiled-coil containing kinase 1
- PAK1:
-
p21-activated kinase 1
- PI3K:
-
Phosphatidylinositide 3-kinase
- JNK:
-
c-Jun N-terminal kinase
References
Cloud J, Kelly CP (2007) Update on Clostridium difficile associated disease. Curr Opin Gastroenterol 23:4–9
Pothoulakis C, Lamont JT (2001) Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol 280:G178–G183
Lyerly DM, Krivan HC, Wilkins TD (1988) Clostridium difficile: its disease and toxins. Clin Microbiol Rev 1:1–18
Sun X, Savidge T, Feng H (2010) The enterotoxicity of Clostridium difficile toxins. Toxins (Basel) 2:1848–1880
Pruitt RN, Lacy DB (2012) Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol 2:1–28
Voth DE, Ballard JD (2005) Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18:247–263
Lyras D, O’Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI (2009) Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179
Aktories K, Just I (2005) Clostridial Rho-inhibiting protein toxins. Curr Top Microbiol Immunol 291:113–145
Huelsenbeck J, Dreger S, Gerhard R, Barth H, Just I, Genth H (2007) Difference in the cytotoxic effects of toxin B from Clostridium difficile strain VPI 10463 and Toxin B from variant Clostridium difficile strain 1470. Infect Immun 75:801–809
Genth H, Huelsenbeck J, Hartmann B, Hofmann F, Just I, Gerhard R (2006) Cellular stability of Rho-GTPases glucosylated by Clostridium difficile toxin B. FEBS Lett 580:3565–3569
Halabi-Cabezon I, Huelsenbeck J, May M, Ladwein M, Rottner K, Just I, Genth H (2008) Prevention of the cytopathic effect induced by Clostridium difficile toxin B by active Rac1. FEBS Lett 582:3751–3756
Popoff MR, Geny B (2011) Rho/Ras-GTPase-dependent and -independent activity of clostridial glucosylating toxins. J Med Microbiol 60:1057–1069
Bishop AL, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348:241–255
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–635
Karlsson R, Pedersen ED, Wang Z, Brakebusch C (2009) Rho GTPase function in tumorigenesis. Biochim Biophys Acta 1796:91–98
Fiorentini C, Arancia G, Paradisi S, Donelli G, Giuliano M, Piemonte F, Mastrantonio P (1989) Effects of Clostridium difficile Toxins A and B on cytoskeleton organization in HEp-2 cells: a comparative morphological study. Toxicon 27:1209–1218
Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont JT, Wenzi E (1995) Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest 95:2004–2011
Gerhard R, Nottrott S, Schoentaube J, Tatge H, Olling A, Just I (2008) Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J Med Microbiol 57:765–770
Fiorentini C, Fabbri A, Falzano L, Fattorossi A, Matarrese P, Rivabene R, Donelli G (1998) Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect Immun 66:2660–2665
Brito GA, Fujji J, Carneiro-Filho BA, Lima AA, Obrig T, Guerrant RL (2002) Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J Infect Dis 186:1438–1447
Mahida YR, Galvin A, Makh S, Hyde S, Sanfilippo L, Borriello SP, Sewell HF (1998) Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect Immun 66:5462–5469
He D, Hagen SJ, Pothoulakis C, Chen M, Medina ND, Warny M, LaMont JT (2000) Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119:139–150
Linseman D, Laessig T, Meintzer MK, McClure M, Barth H, Aktories K, Heidenreich KA (2001) An essential role for Rac/Cdc42 GTPases in cerebellar granule neuron survival. J Biol Chem 276:39123–39131
Hippenstiel S, Schmeck B, N’Guessan PD, Seybold J, Krull M, Preissner K, Eichel-Streiber CV, Suttorp N (2002) Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am J Physiol Lung Cell Mol Physiol 283:L830–L838
Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT (2005) Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875–1888
Matarrese P, Falzano L, Fabbri A, Gambardella L, Frank C, Geny B, Popoff MR, Malorni W, Fiorentini C (2007) Clostridium difficile toxin B causes apoptosis in epithelial cells by thrilling mitochondria. Involvement of ATP-sensitive mitochondrial potassium channels. J Biol Chem 282:9029–9041
Nottrott S, Schoentaube J, Genth H, Just I, Gerhard R (2007) Clostridium difficile toxin A-induced apoptosis is p53-independent but depends on glucosylation of Rho GTPases. Apoptosis 12:1443–1453
Qa’Dan M, Ramsey M, Daniel J, Spyres LM, Safiejko-Mroczka B, Ortiz-Leduc W, Ballard JD (2002) Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell Microbiol 4:425–434
Savidge TC, Pan WH, Newman P, O’Brien M, Anton PM, Pothoulakis C (2003) Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125:413–420
Yu YB, Li YQ (2014) Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol 20:11273–11280
Neunlist M, Rolli-Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P, De Giorgio R (2014) Enteric glial cells: recent developments and future directions. Gastroenterology 147:1230–1237
Gulbransen BD (2014) Enteric glia. Colloquium series on neuroglia in biology and medicine: from physiology to disease. Morgan & Claypool Publishers, San Rafael, pp 1–70
Gulbransen BD, Sharkey KA (2012) Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9:625–632
Cirillo C, Sarnelli G, Esposito G, Turco F, Steardo L, Cuomo R (2011) S100B protein in the gut: the evidence for enteroglial-sustained intestinal inflammation. World J Gastroenterol 17:1261–1266
Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B (2006) The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 55:41–46
Bassotti G, Villanacci V (2011) Can “functional” constipation be considered as a form of enteric neuro-gliopathy? Glia 59:345–350
Rühl A, Trotter J, Stremmel W (2001) Isolation of enteric glia and establishment of transformed enteroglial cell lines from the myenteric plexus of adult rat. Neurogastroenterol Motil 13:95–106
Soret R, Coquenlorge S, Cossais F, Meurette G, Rolli-Derkinderen M, Neunlist M (2013) Characterization of human, mouse, and rat cultures of enteric glial cells and their effect on intestinal epithelial cells. Neurogastroenterol Motil 25:e755–e764
Fettucciari K, Rosati E, Scaringi L, Cornacchione P, Migliorati G, Sabatini R, Fetriconi I, Rossi R, Marconi P (2000) Group B Streptococcus induces apoptosis in macrophages. J Immunol 165:3923–3933
Fettucciari K, Fetriconi I, Mannucci R, Nicoletti I, Bartoli A, Coaccioli S, Marconi P (2006) Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol 176:7542–7556
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) J Immunol Methods 139:271–279
Gerashchenko BI, Azzam EI, Howell RW (2004) Cytometry A 61:134–141
von Boyen GBT (2004) Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53:222–228
May M, Wang T, Müller M, Genth H (2013) Difference in F-actin depolymerization induced by toxin B from the Clostridium difficile strain VPI 10463 and toxin B from the variant Clostridium difficile serotype F strain 1470. Toxins (Basel) 5:106–119
Vermeulen K, Berneman ZN, Van Bockstaele DR (2003) Cell-cycle and apoptosis. Cell Prolif 36:165–175
Jacotot E, Ferri KF, Kroemer G (2000) Apoptosis and cell cycle: distinct checkpoints with overlapping upstream control. Pathol Biol (Paris) 48:271–279
Su CC, Lin JG, Chen GW, Lin WC, Chung JG (2006) Down-regulation of Cdc25c, CDK1 and cyclin B1 and up-regulation of Wee1 by curcumin promotes human colon cancer colo 205 cell entry into G2/M-phase of cell cycle. Cancer Genom Proteom 3:55–62
Zhang XH, Zou ZQ, Xu CW, Shen YZ, Li D (2011) Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting the activity of the cyclin B/Cdc2 complex. Mol Med Rep 4:273–277
Ando Y, Yasuda S, Oceguera-Yanez F, Narumiya S (2007) Inactivation of Rho GTPases with Clostridium difficile toxin B impairs centrosomal activation of Aurora-A in G2/M transition of HeLa cells. Mol Biol Cell 18:3752–3763
Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30:630–641
Wyllie AH (2010) “Where, O death, is thy sting?” A brief review of apoptosis biology. Mol Neurobiol 42:4–9
Kaufmann SH, Hengartner MO (2001) Programmed cell death: alive and well in the new millennium. Trends Cell Biol 11:526–534
Street CA, Bryan BA (2011) Rho kinase proteins—pleiotropic modulators of cell survival and apoptosis. Anticancer Res 31:3645–3657
Schoentaube J, Olling A, Tatge H, Just I, Gerhard R (2009) Serine-71 phosphorylation of Rac1/Cdc42 diminishes the pathogenic effect of Clostridium difficile toxin A. Cell Microbiol 11:1816–1826
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101:7618–7623
Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, Zhang B (2010) Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther 9:1657–1668
Stankiewicz TR, Ramaswami SA, Bouchard RJ, Aktories K, Linseman DA (2015) Neuronal apoptosis induced by selective inhibition of Rac GTPase versus global suppression of Rho family GTPases is mediated by alterations in distinct mitogen-activated protein kinase signaling cascades. J Biol Chem 290:9363–9376
Steele J, Chen K, Sun X, Zhang Y, Wang H, Tzipori S, Feng H (2012) Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J Infect Dis 205:384–391
Czepiel J, Biesiada G, Brzozowski T, Ptak-Belowska A, Perucki W, Birczynska M, Jurczyszyn A, Strzalka M, Targosz A, Garlicki A (2014) The role of local and systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol 65:695–703
Steinkamp M, Schulte N, Spaniol U, Pflüger C, Hartmann C, Kirsch J, von Boyen GB (2012) Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation. Med Sci Monit 18:BR117–BR122
Steinkamp M, Gundel H, Schulte N, Spaniol U, Pflueger C, Zizer E, von Boyen GB (2012) GDNF protects enteric glia from apoptosis: evidence for an autocrine loop. BMC Gastroenterol 12:1–6
Shi J, Wei L (2007) Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 55:61–75
Coqueret O (2003) New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13:65–70
Katayose Y, Kim M, Rakkar AN, Li Z, Cowan KH, Seth P (1997) Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res 57:5441–5445
Wang X, Gorospe M, Huang Y, Holbrook NJ (1997) p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene 15:2991–2997
Kim HJ, Ghil KC, Kim MS, Yeo SH, Chun YJ, Kim MY (2005) Potentiation of ceramide-induced apoptosis by p27kip1 overexpression. Arch Pharm Res 28:87–92
Besson A, Dowdy SF, Roberts JM (2008) CDK inhibitors: cell cycle regulators and beyond. Dev Cell 14:159–169
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394
Xiao W, Wang W, Chen W, Sun L, Li X, Zhang C, Yang H (2014) GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol Neurobiol 50:274–289
Sariola H, Saarma M (2003) Novel functions and signalling pathways for GDNF. J Cell Sci 116:3855–3862
De Giorgio R, Giancola F, Boschetti E, Abdo H, Lardeux B, Neunlist M (2012) Enteric glia and neuroprotection: basic and clinical aspects. Am J Physiol Gastrointest Liver Physiol 303:G887–G893
Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL (2016) Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory Bowel diseases. Inflamm Bowel Dis 22:433–449
Sethi S, Garey KW, Arora V, Ghantoji S, Rowan P, Smolensky M, DuPont HL (2011) Increased rate of irritable bowel syndrome and functional gastrointestinal disorders after Clostridium difficile infection. J Hosp Infect 77:172–173
Piche T, Vanbiervliet G, Pipau FG, Dainese R, Hébuterne X, Rampal P, Collins SM (2007) Low risk of irritable bowel syndrome after Clostridium difficile infection. Can J Gastroenterol 21:727–731
Wadhwa A, Al Nahhas MF, Dierkhising RA, Patel R, Kashyap P, Pardi DS, Khanna S, Grover M (2016) High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther 44:576–582
Acknowledgements
The authors thank Springer Nature Author Services for editing of English language. We are greatly indebted to Alfa Wasserman, Sofar, and Almirall companies for their generous unrestricted support. This work was supported by a Project from the Department of Experimental Medicine for Basic Research (bando 2014) awarded to Doctor Katia Fettucciari, a Grant from Fondazione Cassa di Risparmio di Perugia, Italy (bando 2015-2015.0327.021 Ricerca Scientifica e Tecnologica) to Professor Lanfranco Corazzi, and a grant from “AIGO fa RICERCA” awarded to Professor Gabrio Bassotti by the Italian Society of Hospital Gastroenterologists and Endoscopists (AIGO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fettucciari, K., Ponsini, P., Gioè, D. et al. Enteric glial cells are susceptible to Clostridium difficile toxin B. Cell. Mol. Life Sci. 74, 1527–1551 (2017). https://doi.org/10.1007/s00018-016-2426-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2426-4