Abstract
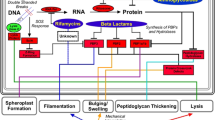
Efforts to reduce the global burden of bacterial disease and contend with escalating bacterial resistance are spurring innovation in antibacterial drug and biocide development and related technologies such as photodynamic therapy and photochemical disinfection. Elucidation of the mechanism of action of these new agents and processes can greatly facilitate their development, but it is a complex endeavour. One strategy that has been popular for many years, and which is garnering increasing interest due to recent technological advances in microscopy and a deeper understanding of the molecular events involved, is the examination of treated bacteria for changes to their morphology and ultrastructure. In this review, we take a critical look at this approach. Variables affecting antibacterial-induced alterations are discussed first. These include characteristics of the test organism (e.g. cell wall structure) and incubation conditions (e.g. growth medium osmolarity). The main body of the review then describes the different alterations that can occur. Micrographs depicting these alterations are presented, together with information on agents that induce the change, and the sequence of molecular events that lead to the change. We close by highlighting those morphological and ultrastructural changes which are consistently induced by agents sharing the same mechanism (e.g. spheroplast formation by peptidoglycan synthesis inhibitors) and explaining how changes that are induced by multiple antibacterial classes (e.g. filamentation by DNA synthesis inhibitors, FtsZ disruptors, and other types of agent) can still yield useful mechanistic information. Lastly, recommendations are made regarding future study design and execution.

Images from [97] by permission of Antimicrobial Agents and Chemotherapy (ASM)

Images from [101] by permission of Innate Immunity (SAGE Publications)

Images from [125] by permission of Antimicrobial Agents and Chemotherapy (ASM)

Images from [155] by permission of Reviews of Infectious Diseases (Oxford University Press)

Images from [195] by permission of the Journal of Medical Microbiology (MicroSoc)

Images from [195] by permission of the Journal of Medical Microbiology (MicroSoc)

Images from [202] by permission of Proceedings of the Society for Experimental Biology and Medicine (SAGE Publications)

Images from [203] by permission of the Journal of Antimicrobial Chemotherapy (Oxford University Press)
Similar content being viewed by others
References
Jessney B (2012) Joseph Lister (1827–1912): a pioneer of antiseptic surgery remembered a century after his death. J Med Biogr 20:107–110
McGuire MJ (2013) The chlorine revolution: water disinfection and the fight to save lives. American Water Works Association, Denver
Greenwood D (2010) Chapter 1: Historical introduction. In: Finch RG et al (eds) Antibiotic and chemotherapy: anti-infective agents and their use in therapy, 9th edn. Elsevier Health Sciences, London, pp 2–9
Fraise AP, Maillard J-Y, Sattar SA (eds) (2012) Russell, Hugo and Ayliffe’s principles and practice of disinfection, preservation, and sterilization, 5th edn. Wiley-Blackwell, Chichester
Cushnie TPT, Cushnie B, Lamb AJ (2014) Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents 44:377–386
Cavera VL, Arthur TD, Kashtanov D, Chikindas ML (2015) Bacteriocins and their position in the next wave of conventional antibiotics. Int J Antimicrob Agents 46:494–501
Pendleton JN, Gilmore BF (2015) The antimicrobial potential of ionic liquids: a source of chemical diversity for infection and biofilm control. Int J Antimicrob Agents 46:131–139
Vo HT, Imai T, Ho TT, Dang T-LT, Hoang SA (2015) Potential application of high pressure carbon dioxide in treated wastewater and water disinfection: recent overview and further trends. J Environ Sci 36:38–47
Yu Q, Wu Z, Chen H (2015) Dual-function antibacterial surfaces for biomedical applications. Acta Biomater 16:1–13
Cloutier M, Mantovani D, Rosei F (2015) Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol 33:637–652
Huang Y-Y, Sharma SK, Yin R, Agrawal T, Chiang LY, Hamblin MR (2014) Functionalized fullerenes in photodynamic therapy. J Biomed Nanotechnol 10:1918–1936
Robertson PKJ, Robertson JMC, Bahnemann DW (2012) Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J Hazard Mater 211–212:161–171
Byrne JA, Dunlop PSM, Hamilton JWJ, Fernández-Ibáñez P, Polo-López I, Sharma PK, Vennard ASM (2015) A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 20:5574–5615
Morente EO, Fernández-Fuentes MA, Burgos MJG, Abriouel H, Pulido RP, Gálvez A (2013) Biocide tolerance in bacteria. Int J Food Microbiol 162:13–25
Silver LL (2011) Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109
Auerbach T, Mermershtain I, Davidovich C, Bashan A, Belousoff M, Wekselman I, Zimmerman E et al (2010) The structure of ribosome–lankacidin complex reveals ribosomal sites for synergistic antibiotics. Proc Natl Acad Sci USA 107:1983–1988
Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ (2010) Challenges of antibacterial discovery revisited. Ann NY Acad Sci 1213:5–19
Linley E, Denyer SP, McDonnell G, Simons C, Maillard J-Y (2012) Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother 67:1589–1596
Zinn J, Jenkins JB, Swofford V, Harrelson B, McCarter S (2010) Intraoperative patient skin prep agents: is there a difference? AORN J 92:662–674
Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP (2010) Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb Drug Resist 16:91–104
Maillard J-Y, Bloomfield S, Coelho JR, Collier P, Cookson B, Fanning S, Hill A et al (2013) Does microbicide use in consumer products promote antimicrobial resistance? A critical review and recommendations for a cohesive approach to risk assessment. Microb Drug Resist 19:344–354
Oldfield E, Feng X (2014) Resistance-resistant antibiotics. Trends Pharmacol Sci 35:664–674
Gnanadhas DP, Marathe SA, Chakravortty D (2013) Biocides—resistance, cross-resistance mechanisms and assessment. Expert Opin Investig Drugs 22:191–206
Franklin T, Snow G (2005) Chapter 3: Antimicrobial agents and cell membranes. Biochemistry and molecular biology of antimicrobial drug action, 6th edn. Springer, New York, pp 47–64
Karaosmanoglu K, Sayar NA, Kurnaz IA, Akbulut BS (2014) Assessment of berberine as a multi-target antimicrobial: a multi-omics study for drug discovery and repositioning. OMICS 18:42–53
Chileveru HR, Lim SA, Chairatana P, Wommack AJ, Chiang I-L, Nolan EM (2015) Visualizing attack of Escherichia coli by the antimicrobial peptide human defensin 5. Biochemistry 54:1767–1777
Sun D, Zhang W, Lv M, Yang E, Zhao Q, Wang W (2015) Antibacterial activity of ruthenium(II) polypyridyl complex manipulated by membrane permeability and cell morphology. Bioorg Med Chem Lett 25:2068–2073
Valotteau C, Calers C, Casale S, Berton J, Stevens CV, Babonneau F, Pradier C-M et al (2015) Biocidal properties of a glycosylated surface: sophorolipids on Au(111). ACS Appl Mater Inter 7:18086–18095
Saritha K, Rajesh A, Manjulatha K, Setty OH, Yenugu S (2015) Mechanism of antibacterial action of the alcoholic extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera (Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br. ex Schult. Front Microbiol 6:Article 00577
Elnakady YA, Chatterjee I, Bischoff M, Rohde M, Josten M, Sahl H-G, Herrmann M et al (2016) Investigations to the antibacterial mechanism of action of kendomycin. PLoS One 11:Article e0146165
O’Driscoll NH, Labovitiadi O, Cushnie TPT, Matthews KH, Mercer DK, Lamb AJ (2013) Production and evaluation of an antimicrobial peptide-containing wafer formulation for topical application. Curr Microbiol 66:271–278
Elie CR, David G, Schmitzer AR (2015) Strong antibacterial properties of anion transporters: a result of depolarization and weakening of the bacterial membrane. J Med Chem 58:2358–2366
Rath G, Hussain T, Chauhan G, Garg T, Goyal AK (2016) Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater Sci Eng C Mater Biol Appl 58:242–253
Longo G, Kasas S (2014) Effects of antibacterial agents and drugs monitored by atomic force microscopy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 6:230–244
Yao Z, Kahne D, Kishony R (2012) Distinct single-cell morphological dynamics under beta-lactam antibiotics. Mol Cell 48:705–712
Peach KC, Bray WM, Winslow D, Linington PF, Linington RG (2013) Mechanism of action-based classification of antibiotics using high-content bacterial image analysis. Mol BioSyst 9:1837–1848
Braga PC, Sala MT, Dal Sasso M (1999) Pharmacodynamic effects of subinhibitory concentrations of rufloxacin on bacterial virulence factors. Antimicrob Agents Chemother 43:1013–1019
Hwang D, Lim Y-H (2015) Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci Rep 5:Article 10029
Waisbren SJ, Hurley DJ, Waisbren BA (1980) Morphological expressions of antibiotic synergism against Pseudomonas aeruginosa as observed by scanning electron microscopy. Antimicrob Agents Chemother 18:969–975
De Oliveira-Garcia D, Dall’Agnol M, Rosales M, Azzuz ACGS, Alcántara N, Martinez MB, Girón JA (2003) Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell Microbiol 5:625–636
Rabanal F, Grau-Campistany A, Vila-Farrés X, Gonzalez-Linares J, Borràs M, Vila J, Manresa A et al (2015) A bioinspired peptide scaffold with high antibiotic activity and low in vivo toxicity. Sci Rep 5:Article 10558
Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS (2010) Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother 54:3132–3142
Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW (2015) Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 70:2456–2464
Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K (1998) Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother 42:199–209
Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, Gemmell CG et al (2003) Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol 41:5–14
Hyo Y, Yamada S, Harada T (2008) Characteristic cell wall ultrastructure of a macrolide-resistant Staphylococcus capitis strain isolated from a patient with chronic sinusitis. Med Mol Morphol 41:160–164
Alsteens D, Verbelen C, Dague E, Raze D, Baulard A, Dufrêne Y (2008) Organization of the mycobacterial cell wall: a nanoscale view. Pflugers Arch 456:117–125
Elliott TSJ, Shelton A, Greenwood D (1987) The response of Escherichia coli to ciprofloxacin and norfloxacin. J Med Microbiol 23:83–88
Chen K, Sun GW, Chua KL, Gan Y-H (2005) Modified virulence of antibiotic-induced Burkholderia pseudomallei filaments. Antimicrob Agents Chemother 49:1002–1009
Braga PC, Dal Sasso M, Maci S (1997) Cefodizime: effects of sub-inhibitory concentrations on adhesiveness and bacterial morphology of Staphylococcus aureus and Escherichia coli: comparison with cefotaxime and ceftriaxone. J Antimicrob Chemother 39:79–84
Dulaney EL, Marx LM (1971) A folic acid linked system in bacterial cell wall synthesis? J Antibiot (Tokyo) 24:713–714
Wojnicz D, Kłak M, Adamski R, Jankowski S (2007) Influence of subinhibitory concentrations of amikacin and ciprofloxacin on morphology and adherence ability of uropathogenic strains. Folia Microbiol 52:429–436
Chang T-W, Weinstein L (1964) Morphological changes in Gram-negative bacilli exposed to cephalothin. J Bacteriol 88:1790–1797
Horii T, Kobayashi M, Sato K, Ichiyama S, Ohta M (1998) An in vitro study of carbapenem-induced morphological changes and endotoxin release in clinical isolates of Gram-negative bacilli. J Antimicrob Chemother 41:435–442
Elliott TSJ, Greenwood D (1983) The response of Pseudomonas aeruginosa to azlocillin, ticarcillin and cefsulodin. J Med Microbiol 16:351–362
Diver JM, Wise R (1986) Morphological and biochemical changes in Escherichia coli after exposure to ciprofloxacin. J Antimicrob Chemother 18:31–41
Bayer M (1967) The cell wall of Escherichia coli: early effects of penicillin treatment and deprivation of diaminopimelic acid. J Gen Microbiol 46:237–246
El-Hajj ZW, Newman EB (2015) How much territory can a single E. coli cell control? Front Microbiol 6:Article 309
Higgins ML, Shockman GD (1970) Early changes in the ultrastructure of Streptococcus faecalis after amino acid starvation. J Bacteriol 103:244–253
Giesbrecht P, Kersten T, Maidhof H, Wecke J (1998) Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol Mol Biol Rev 62:1371–1414
Klainer AS, Perkins RL (1972) Surface manifestations of antibiotic-induced alterations in protein synthesis in bacterial cells. Antimicrob Agents Chemother 1:164–170
Gottfreðsson M, Erlendsdóttir H, Kolka R, Gudmundsson S (1991) Metabolic and ultrastructural effects induced by ciprofloxacin in Staphylococcus aureus during the postantibiotic effect (PAE) phase. Scand J Infect Dis Suppl 74:124–128
De Pedro MA, Donachie WD, Höltje JV, Schwarz H (2001) Constitutive septal murein synthesis in Escherichia coli with impaired activity of the morphogenetic proteins RodA and penicillin-binding protein 2. J Bacteriol 183:4115–4126
Jacquet T, Cailliez-Grimal C, Francius G, Borges F, Imran M, Duval JFL, Revol-Junelles A-M (2012) Antibacterial activity of class IIa bacteriocin Cbn BM1 depends on the physiological state of the target bacteria. Res Microbiol 163:323–331
Dienes L (1949) The development of Proteus cultures in the presence of penicillin. J Bacteriol 57:529–546
Fleming A, Voureka A, Kramer IRH, Hughes WH (1950) The morphology and motility of Proteus vulgaris and other organisms cultured in the presence of penicillin. J Gen Microbiol 4:257–269
Ellis L, Herron D, Preston D, Simmons L, Schlegel R (1976) Evaluation of antibiotic efficacy using electron microscopy: morphological effects of guanylureido cephalosporin, chlorobenzoylureido cephalosporin, BL-P1654, and carbenicillin on Pseudomonas aeruginosa. Antimicrob Agents Chemother 9:334–342
Greenwood D, O’Grady F (1973) FL 1060: a new beta-lactam antibiotic with novel properties. J Clin Pathol 26:1–6
Fitz-James P, Hancock R (1965) The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol 26:657–667
Gebicki J, James A (1960) The preparation and properties of spheroplasts of Aerobacter aerogenes. J Gen Microbiol 23:9–18
Lederberg J (1956) Bacterial protoplasts induced by penicillin. Proc Natl Acad Sci USA 42:574–577
Weibull C (1953) The isolation of protoplasts from Bacilllus megaterium by controlled treatment with lysozyme. J Bacteriol 66:688–695
Silver LL (2012) Rational approaches to antibacterial discovery: pre-genomic directed and phenotypic screening. In: Dougherty TJ, Pucci MJ (eds) Antibiotic discovery and development. Springer, New York, pp 33–75
Van Rensburg AJ (1969) Properties of Proteus mirabilis and Providence spheroplasts. J Gen Microbiol 56:257–264
Spratt BG (1975) Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA 72:2999–3003
Curtis N, Orr D, Ross GW, Boulton MG (1979) Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother 16:533–539
Kitano K, Tomasz A (1979) Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother 16:838–848
Dalhoff A, Nasu T, Okamoto K (2003) Target affinities of faropenem to and its impact on the morphology of Gram-positive and Gram-negative bacteria. Chemotherapy 49:172–183
Greenwood D, O’Grady F (1969) A comparison of the effects of ampicillin on Escherichia coli and Proteus mirabilis. J Med Microbiol 2:435–441
Chin WL, Lawson JW (1976) Effect of antibiotics on L-form induction of Neisseria meningitidis. Antimicrob Agents Chemother 9:1056–1065
Zimmerman SB, Stapley EO (1976) Relative morphological effects induced by cefoxitin and other beta-lactam antibiotics in vitro. Antimicrob Agents Chemother 9:318–326
Nakao M, Nishi T, Tsuchiya K (1981) In vitro and in vivo morphological response of Klebsiella pneumoniae to cefotiam and cefazolin. Antimicrob Agents Chemother 19:901–910
Rodgers FG, Tzianabos AO, Elliott TSJ (1990) The effect of antibiotics that inhibit cell-wall, protein, and DNA synthesis on the growth and morphology of Legionella pneumophila. J Med Microbiol 31:37–44
Nishino T, Nakazawa S (1972) Morphological changes in Staphylococcus aureus and Escherichia coli exposed to cephalexin. Jap J Microbiol 16:83–94
Spratt BG, Cromie KD (1988) Penicillin-binding proteins of Gram-negative bacteria. Rev Infect Dis 10:699–711
Kong K-F, Schneper L, Mathee K (2010) Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS 118:1–36
Hishinuma F, Izaki K, Takahashi H (1971) Inhibition of l-alanine adding enzyme by glycine. Agric Biol Chem 35:2050–2058
Isono F, Katayama T, Inukai M, Haneishi T (1989) Mureidomycins A–D, novel peptidylnucleoside antibiotics with spheroplast forming activity. III. Biological properties. J Antibiot (Tokyo) 42:674–679
Barkhatova OI, Popov VL, Kekcheeva NK, Prozorovskiĭ SV (1984) [Electron microscopic characteristics of the action of penicillin and vancomycin on Rickettsia conorii and Rickettsia akari in vitro] (in Russian). Antibiotiki 29:580–585
Van Heijenoort Y, Leduc M, Singer H, Van Heijenoort J (1987) Effects of moenomycin on Escherichia coli. J Gen Microbiol 133:667–674
Nozaki Y, Katayama N, Harada S, Ono H, Okazaki H (1989) Lactivicin, a naturally occurring non-beta-lactam antibiotic having beta-lactam-like action: biological activities and mode of action. J Antibiot (Tokyo) 42:84–93
Ellison RT 3rd, Giehl TJ (1991) Killing of Gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 88:1080–1091
Nikaido H, Vaara M (1985) Molecular basis of bacterial outer membrane permeability. Microbiol Rev 49:1–32
Birdsell DC, Cota-Robles EH (1967) Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol 93:427–437
Hash JH, Wishnick M, Miller PA (1964) Formation of “protoplasts” of Staphylococcus aureus with a fungal N-acetylhexosaminidase. J Bacteriol 87:432–437
Schuhardt VT, Klesius PH (1968) Osmotic fragility and viability of lysostaphin-induced staphylococcal spheroplasts. J Bacteriol 96:734–737
Klainer AS, Russell RRB (1974) Effect of the inhibition of protein synthesis on the Escherichia coli cell envelope. Antimicrob Agents Chemother 6:216–224
Goss WA, Deitz WH, Cook TM (1964) Mechanism of action of nalidixic acid on Escherichia coli. J Bacteriol 88:1112–1118
Spratt BG, Pardee AB (1975) Penicillin-binding proteins and cell shape in E. coli. Nature 254:516–517
Di Modugno E, Erbetti I, Ferrari L, Galassi G, Hammond SM, Xerri L (1994) In vitro activity of the tribactam GV104326 against Gram-positive, Gram-negative, and anaerobic bacteria. Antimicrob Agents Chemother 38:2362–2368
Jackson JJ, Kropp H (1996) Differences in mode of action of β-lactam antibiotics influence morphology, LPS release and in vivo antibiotic efficacy. J Endotoxin Res 3:201–218
Bernabeu-Wittel M, García-Curiel A, Pichardo C, Pachón-Ibáñez ME, Jiménez-Mejías ME, Pachón J (2004) Morphological changes induced by imipenem and meropenem at sub-inhibitory concentrations in Acinetobacter baumannii. Clin Microbiol Infect 10:931–934
Sumita Y, Fukasawa M, Okuda T (1990) Comparison of two carbapenems, SM-7338 and imipenem: affinities for penicillin-binding proteins and morphological changes. J Antibiot (Tokyo) 43:314–320
Nickerson WJ, Webb M (1956) Effect of folic acid analogues on growth and cell division of nonexacting microorganisms. J Bacteriol 71:129–139
Perumalsamy H, Jung MY, Hong SM, Ahn YJ (2013) Growth-inhibiting and morphostructural effects of constituents identified in Asarum heterotropoides root on human intestinal bacteria. BMC Complement Altern Med 13:Article 245
Spratt BG (1977) Comparison of the binding properties of two 6β-amidinopenicillanic acid derivatives that differ in their physiological effects on Escherichia coli. Antimicrob Agents Chemother 11:161–166
Osborn MJ, Rothfield L (2007) Cell shape determination in Escherichia coli. Curr Opin Microbiol 10:606–610
Nozaki U, Kawashima F, Imada A (1981) C-19393 S2 and H2, new carbapenem antibiotics. III. Mode of action. J Antibiot (Tokyo) 34:206–211
Spratt BG, Jobanputra V, Zimmermann W (1977) Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother 12:406–409
Gutmann L, Vincent S, Billot-Klein D, Acar JF, Mrèna E, Williamson R (1986) Involvement of penicillin-binding protein 2 with other penicillin-binding proteins in lysis of Escherichia coli by some β-lactam antibiotics alone and in synergistic lytic effect of amdinocillin (mecillinam). Antimicrob Agents Chemother 30:906–912
Lorian V, Atkinson B (1977) Comparison of the effects of mecillinam and 6-aminopenicillanic acid on Proteus mirabilis, Escherichia coli, and Staphylococcus aureus. Antimicrob Agents Chemother 11:541–552
Iwai N, Nagai K, Wachi M (2002) Novel S-benzylisothiourea compound that induces spherical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicillin-binding protein 2. Biosci Biotechnol Biochem 66:2658–2662
Bean GJ, Flickinger ST, Westler WM, McCully ME, Sept D, Weibel DB, Amann KJ (2009) A22 disrupts the bacterial actin cytoskeleton by directly binding and inducing a low-affinity state in MreB. Biochemistry 48:4852–4857
Yamachika S, Sugihara C, Tsuji H, Muramatsu Y, Kamai Y, Yamashita M (2012) Anti-Pseudomonas aeruginosa compound, 1,2,3,4-tetrahydro-1,3,5-triazine derivative, exerts its action by primarily targeting MreB. Biol Pharm Bull 35:1740–1744
Wainwright M, Canham LT, Al-Wajeeh K, Reeves CL (1999) Morphological changes (including filamentation) in Escherichia coli grown under starvation conditions on silicon wafers and other surfaces. Lett Appl Microbiol 29:224–227
Braga PC, Dal Sasso M, Sala MT (2000) Sub-MIC concentrations of cefodizime interfere with various factors affecting bacterial virulence. J Antimicrob Chemother 45:15–25
Young KD (2007) Bacterial morphology: why have different shapes? Curr Opin Microbiol 10:596–600
Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ (2004) Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci USA 101:1333–1338
Noguchi H, Matsuhashi M, Takaoka M, Mitsuhashi S (1978) New antipseudomonal penicillin, PC-904: affinity to penicillin-binding proteins and inhibition of the enzyme cross-linking peptidoglycan. Antimicrob Agents Chemother 14:617–624
Tanaka M, Otsuki M, Nishino T (1992) In vitro and in vivo activities of DQ-2556 and its mode of action. Antimicrob Agents Chemother 36:2595–2601
Presslitz JE (1978) Mode of action of a structurally novel beta-lactam. Antimicrob Agents Chemother 14:144–150
Onoe T, Umemoto T, Sagawa H, Suginaka H (1981) Filament formation of Fusobacterium nucleatum cells induced by mecillinam. Antimicrob Agents Chemother 19:487–489
Nakao M, Yukishige K, Kondo M, Imada A (1986) Novel morphological changes in Gram-negative bacteria caused by combination of bulgecin and cefmenoxime. Antimicrob Agents Chemother 30:414–417
Dring GJ, Hurst A (1969) Observations on the action of benzylpenicillin on a strain of Streptococcus lactis. J Gen Microbiol 55:185–194
Lorian V, Atkinson B (1976) Effects of subinhibitory concentrations of antibiotics on cross walls of cocci. Antimicrob Agents Chemother 9:1043–1055
Murphy TF, Barza M, Park JT (1981) Penicillin-binding proteins in Clostridium perfringens. Antimicrob Agents Chemother 20:809–813
Lorian V, Atkinson B (1975) Abnormal forms of bacteria produced by antibiotics. Am J Clin Pathol 64:678–688
Lorian V, Sabath LD (1972) Penicillins and cephalosporins: differences in morphologic effects on Proteus mirabilis. J Infect Dis 125:560–564
Walker JR, Pardee AB (1968) Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol 95:123–131
Ohkawa T (1975) Studies of intracellular thymidine nucleotides. Thymineless death and the recovery after re-addition of thymine in Escherichia coli K12. Eur J Biochem 60:57–66
Chadfield MS, Hinton MH (2004) In vitro activity of nitrofuran derivatives on growth and morphology of Salmonella enterica serotype Enteritidis. J Appl Microbiol 96:1002–1012
Church DL, Bryant RD, Rabin HR, Laishley EJ (1991) Physiolgical effects of metronidazole on Clostridium posteurianum. J Antimicrob Chemother 28:221–228
Suzuki H, Pangborn J, Kilgore WW (1967) Filamentous cells of Escherichia coli formed in the presence of mitomycin. J Bacteriol 93:683–688
Cheng G, Hao H, Dai M, Liu Z, Yuan Z (2013) Antibacterial action of quinolones: from target to network. Eur J Med Chem 66:555–562
Lewin CS, Amyes SGB (1991) The role of the SOS response in bacteria exposed to zidovudine or trimethoprim. J Med Microbiol 34:329–332
Mason D, Power E, Talsania H, Phillips I, Gant V (1995) Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother 39:2752–2758
Ingham CJ, Van Den Ende M, Wever PC, Schneeberger PM (2006) Rapid antibiotic sensitivity testing and trimethoprim-mediated filamentation of clinical isolates of the Enterobacteriaceae assayed on a novel porous culture support. J Med Microbiol 55:1511–1519
Humphrey S, MacVicar T, Stevenson A, Roberts M, Humphrey TJ, Jepson MA (2011) SulA-induced filamentation in Salmonella enterica serovar Typhimurium: effects on SPI-1 expression and epithelial infection. J Appl Microbiol 111:185–196
Ray S, Dhaked HPS, Panda D (2014) Antimicrobial peptide CRAMP (16–33) stalls bacterial cytokinesis by inhibiting FtsZ assembly. Biochemistry 53:6426–6429
Bergersen FJ (1953) Cytological changes induced in Bacterium coli by chloramphenicol. J Gen Microbiol 9:353–356
Elliott TS, Rodgers FG (1985) Morphological response and growth characteristics of Legionella pneumophila exposed to ampicillin and erythromycin. J Med Microbiol 19:383–390
Gilleland LB, Gilleland HE, Gibson JA, Champlin FR (1989) Adaptive resistance to aminoglycoside antibiotics in Pseudomonas aeruginosa. J Med Microbiol 29:41–50
Someya A, Tanaka K, Tanaka N (1979) Morphological changes of Escherichia coli induced by bicyclomycin. Antimicrob Agents Chemother 16:87–91
Paulander W, Wang Y, Folkesson A, Charbon G, Løbner-Olesen A, Ingmer H (2014) Bactericidal antibiotics increase hydroxyphenyl fluorescein signal by altering cell morphology. PLoS One 9:Article e92231
Magnussen CR, Hruska JF (1980) Aberrant forms of Escherichia coli in blood cultures: in vitro reproduction of an in vivo observation. J Clin Microbiol 12:690–694
Morgan C, Rosenkranz HS, Carr HS, Rose HM (1967) Electron microscopy of chloramphenicol-treated Escherichia coli. J Bacteriol 93:1987–2002
Conrad RS, Howard MJ, Garrison RC, Winters S, Henderson DA (1998) The effects of daptomycin on chemical composition and morphology of Staphylococcus aureus. Proc Okla Acad Sci 78:15–22
Neirinck L, DeVoe I (1981) Anomalous cellular morphology and growth characteristics of Neisseria meningitidis in subminimal inhibitory concentrations of penicillin G. Antimicrob Agents Chemother 19:911–916
Tomasz A (1968) Biological consequences of the replacement of choline by ethanolamine in the cell wall of pneumococcus: chain formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci USA 59:86–93
Filice G, Carnevale G, Lanzarini P, Castelli F, Zappala C, Menghini P, De Rysky C (1986) Alterations due to ampicillin and rifampicin in S. sanguis and S. aureus isolated from dental plaque. An electron microscopic study. Chemioterapia 5:3–6
Root RK, Isturiz R, Molavi A, Metcalf JA, Malech HL (1981) Interactions between antibiotics and human neutrophils in the killing of staphylococci: studies with normal and cytochalasin B-treated cells. J Clin Invest 67:247–259
Henze U, Sidow T, Wecke J, Labischinski H, Berger-Bächi B (1993) Influence of femB on methicillin resistance and peptidoglycan metabolism in Staphylococcus aureus. J Bacteriol 175:1612–1620
Sieradzki K, Tomasz A (2006) Inhibition of the autolytic system by vancomycin causes mimicry of vancomycin-intermediate Staphylococcus aureus-type resistance, cell concentration dependence of the MIC, and antibiotic tolerance in vancomycin-susceptible S. aureus. Antimicrob Agents Chemother 50:527–533
Sieradzki K, Tomasz A (2003) Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J Bacteriol 185:7103–7110
Lorian V, Atkinson B, Kim Y (1983) Effect of rifampin and oxacillin on the ultrastructure and growth of Staphylococcus aureus. Rev Infect Dis 5:S418–S427
Nakao M, Kitanaka E, Ochiai K, Nakazawa S (1972) Cell wall synthesis by Staphylococcus aureus in the presence of protein synthesis inhibitory agents. II. Electron microscopic study. J Antibiot (Tokyo) 25:469–470
Richards RME, Xing JZ, Gregory DW, Marshall D (1995) Mechanism of sulphadiazine enhancement of trimethoprim activity against sulphadiazine-resistant Enterococcus faecalis. J Antimicrob Chemother 36:607–618
Lorian V (1975) Some effects of subinhibitory concentrations of penicillin on the structure and division of staphylococci. Antimicrob Agents Chemother 7:864–870
Eirich J, Orth R, Sieber SA (2011) Unraveling the protein targets of vancomycin in living S. aureus and E. faecalis cells. J Am Chem Soc 133:12144–12153
Nakao M, Kitanaka E, Ochiai K, Nakazawa S (1972) Cell wall synthesis by Staphylococcus aureus in the presence of protein synthesis inhibitory agents. I. Electronmicroscopic study. J Antibiot (Tokyo) 25:60–63
Sayare M, Daneo-Moore L, Shockman GD (1972) Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol 112:337–344
Sharma M, Chauhan PM (2012) Dihydrofolate reductase as a therapeutic target for infectious diseases: opportunities and challenges. Future Med Chem 4:1335–1365
Rogers HJ, Taylor C (1978) Autolysins and shape change in rodA mutants of Bacillus subtilis. J Bacteriol 135:1032–1042
Cotroneo N, Harris R, Perlmutter N, Beveridge T, Silverman JA (2008) Daptomycin exerts bactericidal activity without lysis of Staphylococcus aureus. Antimicrob Agents Chemother 52:2223–2225
Cheng M, Huang JX, Ramu S, Butler MS, Cooper MA (2014) Ramoplanin induces bacterial membrane depolarization in Staphylococcus aureus at bactericidal concentrations. Antimicrob Agents Chemother 58:6819–6827
Lutkenhaus J, Pichoff S, Du S (2012) Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton 69:778–790
Tsang KW, Ng P, Ho PL, Chan S, Tipoe G, Leung R, Sun J et al (2003) Effects of erythromycin on Pseudomonas aeruginosa adherence to collagen and morphology in vitro. Eur Respir J 21:401–406
Greenwood D, O’Grady F (1972) Scanning electron microscopy of Staphylococcus aureus exposed to some common anti-staphylococcal agents. J Gen Microbiol 70:263–270
Burdett IDJ, Murray RGE (1974) Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol 119:303–324
Braga PC, Ricci D (2000) Detection of rokitamycin-induced morphostructural alterations in Helicobacter pylori by atomic force microscopy. Chemotherapy 46:15–22
Chan EL, Harris RC, Dalton HP (1987) The effect of antibiotics on the cell morphology of Legionella pneumophila. J Med Microbiol 23:149–154
Huang KC, Mukhopadhyay R, Wen B, Gitai Z, Wingreen NS (2008) Cell shape and cell-wall organization in Gram-negative bacteria. Proc Natl Acad Sci USA 105:192282–192287
Koupal LR, Pelak BA, Cassidy PJ, Gadebusch HH (1983) Quaternary heterocyclylamino β-lactams. III. The mode of action of L-640,876 and the effect of NaCl on membrane permeability and binding. J Antibiot (Tokyo) 36:54–63
Nakao M, Kondo M, Tsuchiya K (1981) Light and electron microscopy of the morphological response of Escherichia coli and Serratia marcescens to cefmenoxime (SCE-1365), a new broad-spectrum cephalosporin. J Antibiot (Tokyo) 34:1046–1054
Daly KE, Huang KC, Wingreen NS, Mukhopadhyay R (2011) Mechanics of membrane bulging during cell-wall disruption in Gram-negative bacteria. Phys Rev E Stat Nonlin Soft Matter Phys 83:Article 041922
Pogliano J, Pogliano N, Silverman JA (2012) Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J Bacteriol 194:4494–4504
Wale LJ, Shelton AP, Greenwood D (1989) Scanning electron microscopy of Staphylococcus aureus and Enterococcus faecalis exposed to daptomycin. J Med Microbiol 30:45–49
Comber KR, Boon RJ, Sutherland R (1977) Comparative effects of amoxycillin and ampicillin on the morphology of Escherichia coli in vivo and correlation with activity. Antimicrob Agents Chemother 12:736–744
Tanaka N, Matsunaga K, Hirata A, Matsuhisa Y, Nishimura T (1983) Mechanism of action of habekacin, a novel amino acid-containing aminoglycoside antibiotic. Antimicrob Agents Chemother 24:797–802
Koike M, Iida K, Matsuo T (1969) Electron microscopic studies on mode of action of polymyxin. J Bacteriol 97:448–452
Martin NL, Beveridge TJ (1986) Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob Agents Chemother 29:1079–1087
Iida K, Koike M (1974) Cell wall alterations of Gram-negative bacteria by aminoglycoside antibiotics. Antimicrob Agents Chemother 5:95–97
Kadurugamuwa JL, Clarke AJ, Beveridge TJ (1993) Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol 175:5798–5805
Schindler PRG, Teuber M (1975) Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother 8:95–104
Nation RL, Velkov T, Li J (2014) Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94
Davis BD, Chen L, Tai PC (1986) Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci USA 83:6164–6168
Hancock RE, Raffle VJ, Nicas TI (1981) Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 19:777–785
Higgins ML, Daneo-Moore L, Boothby D, Shockman GD (1974) Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol 118:681–692
Miller IL, Zsigray RM, Landman OE (1967) The formation of protoplasts and quasi-spheroplasts in normal and chloramphenicol-pretreated Bacillus subtilis. J Gen Microbiol 49:513–525
Molitor E, Kluczny C, Brötz H, Bierbaum G, Jack R, Sahl H-G (1996) Effects of the lantibiotic mersacidin on the morphology of staphylococci. Zentralbl Bakteriol 284:318–328
Nakao M, Kitanaka F, Ochiai K, Nakazwa S (1972) Cell wall synthesis in Staphylococcus aureus in the presence of protein synthesis inhibitory agents. I. Lincomycin, clindamycin and macrolide antibiotics. Jap J Microbiol 16:403–413
Nishino T (1975) An electron microscopic study of antagonism between cephalexin and erythromycin in Staphylococcus aureus. Jap J Microbiol 19:53–63
Hash JH, Davies MC (1962) Electron microscopy of Staphylococcus aureus treated with tetracycline. Science 138:828–829
Wheeler R, Turner R, Bailey R, Salamaga B, Mesnage S, Mohamad S, Hayhurst E et al (2015) Bacterial cell enlargement requires control of cell wall stiffness mediated by peptidoglycan hydrolases. MBio 6:Article e00660–e00615
Richards RME, Xing JZ, Gregory DW, Marshall D (1993) An electron microscope study of the effect of sulphadiazine and trimethoprim on Enterobacter cloacae. J Med Microbiol 38:64–68
Reisner BS, Woods GL, Popov VL (1997) Electron microscopic analysis of Mycobacterium avium complex isolates exposed to ciprofloxacin, rifabutin, ethambutol and clarithromycin. Int J Tuberc Lung Dis 1:270–275
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:Article a000414
Georgiou G, Telford JN, Shuler ML, Wilson DB (1986) Localization of inclusion bodies in Escherichia coli overproducing β-lactamase or alkaline phosphatase. Appl Environ Microbiol 52:1157–1161
Tuomanen E, Gilbert K, Tomasz A (1986) Modulation of bacteriolysis by cooperative effects of penicillin-binding proteins 1a and 3 in Escherichia coli. Antimicrob Agents Chemother 30:659–663
Lorian V, Atkinson BA (1986) Amikacin-induced alterations in the structure of Gram-negative bacilli. Diagn Microbiol Infect Dis 5:93–97
Lorian V, Fernandes F (1998) Effect of quinupristin/dalfopristin alone or in combination with vancomycin on the structure of Enterococcus faecium. Drugs Exp Clin Res 24:73–76
Lorian V, Sabath LD, Simionescu M (1975) Decrease in ribosomal density of Proteus mirabilis exposed to subinhibitory concentrations of ampicillin or cephalothin. Proc Soc Exp Biol Med 149:731–735
Lorian V, Fernandes F (1999) Electron microscopy studies of the bactericidal effects of quinupristin/dalfopristin on Staphylococcus aureus. J Antimicrob Chemother 43:845–846
Formosa C, Grare M, Jauvert E, Coutable A, Regnouf-de-Vains JB, Mourer M, Duval RE et al (2012) Nanoscale analysis of the effects of antibiotics and CX1 on a Pseudomonas aeruginosa multidrug-resistant strain. Sci Rep 2:Article 575
Oliva B, Miller K, Caggiano N, O’Neill AJ, Cuny GD, Hoemann MZ, Hauske JR et al (2003) Biological properties of novel antistaphylococcal quinoline-indole agents. Antimicrob Agents Chemother 47:458–466
Boberek JM, Stach J, Good L (2010) Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS One 5:Article e13745
Acknowledgments
We are very grateful to those authors who helped source the more-difficult-to-find journal articles used in this review, in particular Professors Francesco Castelli, Magnús Gottfreðsson, Giovanni Longo, Vsevolod L. Popov, R. Michael E. Richards, Brian G. Spratt, and Alexander Tomasz. We are also extremely grateful to Colin MacLean and the Interlibrary loan staff at the Robert Gordon University for their assistance with this project, and to Dr. Helen Vosper for constructive comments during drafting of the manuscript. Our apologies to authors whose work could not be included due to space restrictions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cushnie, T.P.T., O’Driscoll, N.H. & Lamb, A.J. Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action. Cell. Mol. Life Sci. 73, 4471–4492 (2016). https://doi.org/10.1007/s00018-016-2302-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2302-2