Abstract
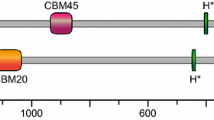
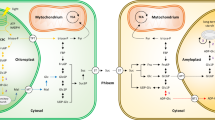
Glucan phosphatases are a family of enzymes that are functionally conserved at the enzymatic level in animals and plants. These enzymes bind and dephosphorylate glycogen in animals and starch in plants. While the enzymatic function is conserved, the glucan phosphatases employ distinct mechanisms to bind and dephosphorylate glycogen or starch. The founding member of the family is a bimodular human protein called laforin that is comprised of a carbohydrate binding module 20 (CBM20) followed by a dual specificity phosphatase domain. Plants contain two glucan phosphatases: Starch EXcess4 (SEX4) and Like Sex Four2 (LSF2). SEX4 contains a chloroplast targeting peptide, dual specificity phosphatase (DSP) domain, a CBM45, and a carboxy-terminal motif. LSF2 is comprised of simply a chloroplast targeting peptide, DSP domain, and carboxy-terminal motif. SEX4 employs an integrated DSP-CBM glucan-binding platform to engage and dephosphorylate starch. LSF2 lacks a CBM and instead utilizes two surface binding sites to bind and dephosphorylate starch. Laforin is a dimeric protein in solution and it utilizes a tetramodular architecture and cooperativity to bind and dephosphorylate glycogen. This chapter describes the biological role of glucan phosphatases in glycogen and starch metabolism and compares and contrasts their ability to bind and dephosphorylate glucans.






Similar content being viewed by others
Abbreviations
- CBM:
-
Carbohydrate binding module
- CGTase:
-
Cyclodextrin glycosyltransferase
- DSP:
-
Dual specificity phosphatase
- GWD:
-
Glucan-water dikinase
- PWD:
-
Phosphoglucan-water dikinase
- LSF2:
-
Like Sex Four2
- SBS:
-
Surface binding site
- SEX4:
-
Starch EXcess4
References
Shearer J, Graham TE (2002) New perspectives on the storage and organization of muscle glycogen. Can J Appl Physiol 27:179–203
Roach PJ (2002) Glycogen and its metabolism. Curr Mol Med 2:101–120
Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS (2012) Glycogen and its metabolism: some new developments and old themes. Biochem J 441:763–787
Ryu JH, Drain J, Kim JH, McGee S, Gray-Weale A, Waddington L, Parker GJ, Hargreaves M, Yoo SH, Stapleton D (2009) Comparative structural analyses of purified glycogen particles from rat liver, human skeletal muscle and commercial preparations. Int J Biol Macromol 45:478–482
Powell PO, Sullivan MA, Sheehy JJ, Schulz BL, Warren FJ, Gilbert RG (2015) Acid hydrolysis and molecular density of phytoglycogen and liver glycogen helps understand the bonding in glycogen alpha (composite) particles. PLoS One 10:e0121337
Goldsmith E, Sprang S, Fletterick R (1982) Structure of maltoheptaose by difference Fourier methods and a model for glycogen. J Mol Biol 156:411–427
Ball S, Colleoni C, Cenci U, Raj JN, Tirtiaux C (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62:1775–1801
Zeeman SC, Kossmann J, Smith AM (2010) Starch: its metabolism, evolution, and biotechnological modification in plants. Ann Rev Plant Biol 61:209–234
Hancock RD, Tarbet BJ (2000) The other double helix—the fascinating chemistry of starch. J Chem Educ 77:988
Ritte G, Heydenreich M, Mahlow S, Haebel S, Kotting O, Steup M (2006) Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett 580:4872–4876
Meekins DA, Raththagala M, Auger KD, Turner BD, Santelia D, Kotting O, Gentry MS, Kooi CWV (2015) Mechanistic insights into glucan phosphatase activity against polyglucan substrates. J Biol Chem 290:23361–23370
Hejazi M, Fettke J, Kotting O, Zeeman SC, Steup M (2010) The Laforin-like dual-specificity phosphatase SEX4 from Arabidopsis hydrolyzes both C6- and C3-phosphate esters introduced by starch-related dikinases and thereby affects phase transition of alpha-glucans. Plant Physiol 152:711–722
Santelia D, Kotting O, Seung D, Schubert M, Thalmann M, Bischof S, Meekins DA, Lutz A, Patron N, Gentry MS, Allain FH, Zeeman SC (2011) The phosphoglucan phosphatase like sex Four2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell. 23:4096–4111
Niittyla T, Comparot-Moss S, Lue WL, Messerli G, Trevisan M, Seymour MDJ, Gatehouse JA, Villadsen D, Smith SM, Chen J, Zeeman SC, Smith AM (2006) Similar protein phosphatases control starch metabolism in plants and glycogen metabolism in mammals. J Biol Chem 281:11815–11818
Zeeman SC, Northrop F, Smith AM, Rees T (1998) A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J. 15:357–365
Kotting O, Santelia D, Edner C, Eicke S, Marthaler T, Gentry MS, Comparot-Moss S, Chen J, Smith AM, Steup M, Ritte G, Zeeman SC (2009) STARCH-EXCESS4 Is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell. 21:334–346
Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Ann Rev Plant Biol 54:207–233
Worby CA, Gentry MS, Dixon JE (2006) Laforin: a dual specificity phosphatase that dephosphorylates complex carbohydrates. J Biol Chem 281:30412–30418
DePaoli-Roach AA, Contreras CJ, Segvich DM, Heiss C, Ishihara M, Azadi P, Roach PJ (2015) Glycogen phosphomonoester distribution in mouse models of the progressive myoclonic epilepsy, Lafora disease. J Biol Chem 290:841–850
Nitschke F, Wang P, Schmieder P, Girard JM, Awrey DE, Wang T, Israelian J, Zhao X, Turnbull J, Heydenreich M, Kleinpeter E, Steup M, Minassian BA (2013) Hyperphosphorylation of glucosyl C6 carbons and altered structure of glycogen in the neurodegenerative epilepsy Lafora disease. Cell Metab 17:756–767
Fordham-Skelton AP, Chilley P, Lumbreras V, Reignoux S, Fenton TR, Dahm CC, Pages M, Gatehouse JA (2002) A novel higher plant protein tyrosine phosphatase interacts with SNF1-related protein kinases via a KIS (kinase interaction sequence) domain. Plant J 29:705–715
Kerk D, Conley TR, Rodriguez FA, Tran HT, Nimick M, Muench DG, Moorhead GB (2006) A chloroplast-localized dual-specificity protein phosphatase in Arabidopsis contains a phylogenetically dispersed and ancient carbohydrate-binding domain, which binds the polysaccharide starch. Plant J. 46:400–413
Gentry MS, Dowen RH 3rd, Worby CA, Mattoo S, Ecker JR, Dixon JE (2007) The phosphatase laforin crosses evolutionary boundaries and links carbohydrate metabolism to neuronal disease. J Cell Biol 178:477–488
Comparot-Moss S, Kotting O, Stettler M, Edner C, Graf A, Weise SE, Streb S, Lue WL, MacLean D, Mahlow S, Ritte G, Steup M, Chen J, Zeeman SC, Smith AM (2010) A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiol 152:685–697
Moorhead GB, De Wever V, Templeton G, Kerk D (2009) Evolution of protein phosphatases in plants and animals. Biochem J 417:401–409
Tonks NK (2013) Protein tyrosine phosphatases–from housekeeping enzymes to master regulators of signal transduction. FEBS J 280:346–378
Alonso A, Rojas A, Godzik A, Mustelin T (2003) The dual-specific protein tyrosine phosphatase family. Springer, Berlin
Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7:833–846
Kerk D, Templeton G, Moorhead GB (2008) Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol 146:351–367
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495
Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Henrissat GDB, Svensson B (eds) Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, pp 3–12
Christiansen C, Abou Hachem M, Janecek S, Vikso-Nielsen A, Blennow A, Svensson B (2009) The carbohydrate-binding module family 20–diversity, structure, and function. FEBS J 276:5006–5029
Janecek S, Svensson B, MacGregor EA (2011) Structural and evolutionary aspects of two families of non-catalytic domains present in starch and glycogen binding proteins from microbes, plants and animals. Enzyme Microb Technol 49:429–440
Janecek S, Blesak K (2011) Sequence-structural features and evolutionary relationships of family GH57 alpha-amylases and their putative alpha-amylase-like homologues. Protein J 30:429–435
Meekins DA, Guo HF, Husodo S, Paasch BC, Bridges TM, Santelia D, Kotting O, Vander Kooi CW, Gentry MS (2013) Structure of the Arabidopsis glucan phosphatase like sex Four2 reveals a unique mechanism for starch dephosphorylation. Plant Cell. 25:2302–2314
Bozonnet S, Jensen MT, Nielsen MM, Aghajari N, Jensen MH, Kramhoft B, Willemoes M, Tranier S, Haser R, Svensson B (2007) The ‘pair of sugar tongs’ site on the non-catalytic domain C of barley alpha-amylase participates in substrate binding and activity. FEBS J 274:5055–5067
Cuyvers S, Dornez E, Delcour JA, Courtin CM (2012) Occurrence and functional significance of secondary carbohydrate binding sites in glycoside hydrolases. Crit Rev Biotechnol 32:93–107
Koropatkin NM, Smith TJ (2010) SusG: a unique cell-membrane-associated alpha-amylase from a prominent human gut symbiont targets complex starch molecules. Structure 18:200–215
Kadziola A, Sogaard M, Svensson B, Haser R (1998) Molecular structure of a barley alpha-amylase-inhibitor complex: implications for starch binding and catalysis. J Mol Biol 278:205–217
Robert X, Haser R, Mori H, Svensson B, Aghajari N (2005) Oligosaccharide binding to barley alpha-amylase 1. J Biol Chem 280:32968–32978
Sevcik J, Hostinova E, Solovicova A, Gasperik J, Dauter Z, Wilson KS (2006) Structure of the complex of a yeast glucoamylase with acarbose reveals the presence of a raw starch binding site on the catalytic domain. FEBS J 273:2161–2171
Gentry MS, Pace RM (2009) Conservation of the glucan phosphatase laforin is linked to rates of molecular evolution and the glycogen metabolism of the organism. BMC Evol Biol 9:138
Ball S, Colleoni C, Arias MC (2015) The transition from glycogen to starch metabolism in cyanobacteria and eukaryotes. In: Nakamura Y (ed) Starch: metabolism and structure. Springer, Japan, pp 93–158
Meekins DA, Raththagala M, Husodo S, White CJ, Guo HF, Kotting O, Vander Kooi CW, Gentry MS (2014) Phosphoglucan-bound structure of starch phosphatase Starch Excess4 reveals the mechanism for C6 specificity. Proc Natl Acad Sci USA 111:7272–7277
Raththagala M, Brewer MK, Parker MW, Sherwood AR, Wong BK, Hsu S, Bridges TM, Paasch BC, Hellman LM, Husodo S, Meekins DA, Taylor AO, Turner BD, Auger KD, Dukhande VV, Chakravarthy S, Sanz P, Woods VL, Li S, Vander Kooi CW, Gentry MS (2015) Structural mechanism of laforin function in glycogen dephosphorylation and lafora disease. Mol Cell 57:261–272
Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94
Vander Kooi CW, Taylor AO, Pace RM, Meekins DA, Guo HF, Kim Y, Gentry MS (2010) From the cover: structural basis for the glucan phosphatase activity of Starch Excess4. Proc Natl Acad Sci USA 107:15379–15384
Barford D, Flint AJ, Tonks NK (1994) Crystal structure of human protein tyrosine phosphatase 1B. Science 263:1397–1404
Yuvaniyama J, Denu JM, Dixon JE, Saper MA (1996) Crystal structure of the dual specificity protein phosphatase VHR. Science 272:1328–1331
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323–334
Holm L, Sander C (1995) Dali: a network tool for protein structure comparison. Trends Biochem Sci 20:478–480
Mobbs JI, Koay A, Di Paolo A, Bieri M, Petrie EJ, Gorman MA, Doughty L, Parker MW, Stapleton DI, Griffin MD, Gooley PR (2015) Determinants of oligosaccharide specificity of the carbohydrate-binding modules of AMP-activated protein kinase. Biochem J 468:245–257
Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW (2005) Structural basis for glycogen recognition by AMP-activated protein kinase. Structure 13:1453–1462
Nielsen MM, Bozonnet S, Seo ES, Motyan JA, Andersen JM, Dilokpimol A, Abou Hachem M, Gyemant G, Naested H, Kandra L, Sigurskjold BW, Svensson B (2009) Two secondary carbohydrate binding sites on the surface of barley alpha-amylase 1 have distinct functions and display synergy in hydrolysis of starch granules. Biochemistry 48:7686–7697
Payan F, Qian M (2003) Crystal structure of the pig pancreatic alpha-amylase complexed with malto-oligosaccharides. J Protein Chem 22:275–284
Ragunath C, Manuel SG, Venkataraman V, Sait HB, Kasinathan C, Ramasubbu N (2008) Probing the role of aromatic residues at the secondary saccharide-binding sites of human salivary alpha-amylase in substrate hydrolysis and bacterial binding. J Mol Biol 384:1232–1248
Bott R, Saldajeno M, Cuevas W, Ward D, Scheffers M, Aehle W, Karkehabadi S, Sandgren M, Hansson H (2008) Three-dimensional structure of an intact glycoside hydrolase family 15 glucoamylase from Hypocrea jecorina. Biochemistry 47:5746–5754
Uitdehaag JC, Mosi R, Kalk KH, van der Veen BA, Dijkhuizen L, Withers SG, Dijkstra BW (1999) X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the alpha-amylase family. Nat Struct Biol 6:432–436
Penninga D, van der Veen BA, Knegtel RM, van Hijum SA, Rozeboom HJ, Kalk KH, Dijkstra BW, Dijkhuizen L (1996) The raw starch binding domain of cyclodextrin glycosyltransferase from Bacillus circulans strain 251. J Biol Chem 271:32777–32784
Dukhande VV, Rogers DM, Roma-Mateo C, Donderis J, Marina A, Taylor AO, Sanz P, Gentry MS (2011) Laforin, a dual specificity phosphatase involved in Lafora disease, is present mainly as monomeric form with full phosphatase activity. PLoS One 6:e24040
Koksal AC, Cingolani G (2011) Dimerization of vaccinia virus VH1 is essential for dephosphorylation of STAT1 at tyrosine 701. J Biol Chem 286:14373–14382
Liu Y, Wang Y, Wu C, Liu Y, Zheng P (2006) Dimerization of Laforin is required for its optimal phosphatase activity, regulation of GSK3beta phosphorylation, and Wnt signaling. J Biol Chem 281:34768–34774
Liu Y, Wang Y, Wu C, Zheng P (2009) Deletions and missense mutations of EPM2A exacerbate unfolded protein response and apoptosis of neuronal cells induced by endoplasm reticulum stress. Hum Mol Genet 18:2622–2631
Sanchez-Martin P, Raththagala M, Bridges TM, Husodo S, Gentry MS, Sanz P, Roma-Mateo C (2013) Dimerization of the glucan phosphatase laforin requires the participation of cysteine 329. PLoS One 8:e69523
Sankhala RS, Koksal AC, Ho L, Nitschke F, Minassian BA, Cingolani G (2015) Dimeric quaternary structure of human laforin. J Biol Chem 290:4552–4559
Jacques DA, Trewhella J (2010) Small-angle scattering for structural biology—expanding the frontier while avoiding the pitfalls. Protein Sci Publ Protein Soc 19:642–657
Schneidman-Duhovny D, Kim SJ, Sali A (2012) Integrative structural modeling with small angle X-ray scattering profiles. BMC Struct Biol 12:17
Wilkens C, Auger KD, Anderson NT, Meekins DA, Raththagala M, Abou Hachem M, Payne CM, Gentry MS, Svensson B (2016) Plant alpha-glucan phosphatases SEX4 and LSF2 display different affinity for amylopectin and amylose. FEBS Lett 590:118–128
Mahlow S, Hejazi M, Kuhnert F, Garz A, Brust H, Baumann O, Fettke J (2014) Phosphorylation of transitory starch by alpha-glucan, water dikinase during starch turnover affects the surface properties and morphology of starch granules. New Phytol 203:495–507
Skeffington AW, Graf A, Duxbury Z, Gruissem W, Smith AM (2014) Glucan, water dikinase exerts little control over starch degradation in arabidopsis leaves at night. Plant Physiol 165:866–879
Tagliabracci VS, Heiss C, Karthik C, Contreras CJ, Glushka J, Ishihara M, Azadi P, Hurley TD, DePaoli-Roach AA, Roach PJ (2011) Phosphate incorporation during glycogen synthesis and Lafora disease. Cell Metab 13:274–282
Acknowledgments
This work was supported by National Institutes of Health Grants R01NS070899; Kentucky Science and Engineering Foundation Grants KSEF-2268RDE-014 and KSEF-2971-RDE-017; Mitzutani Foundation for Glycoscience Award; National Science Foundation Grants IIA-1355438 and MCB-1252345. M. S. G. is co-founder of OptiMol Enzymes LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Emanuelle and M. K. Brewer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Emanuelle, S., Brewer, M.K., Meekins, D.A. et al. Unique carbohydrate binding platforms employed by the glucan phosphatases. Cell. Mol. Life Sci. 73, 2765–2778 (2016). https://doi.org/10.1007/s00018-016-2249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2249-3