Abstract
NLRs (nucleotide-binding domain, leucine-rich repeat containing receptors) are pattern recognition receptors associated with immunity and inflammation in response to endogenous and exogenous pathogen and damage associated molecular patterns (PAMPs and DAMPs respectively). Dysregulated NLR function is associated with several diseases including cancers, metabolic diseases, autoimmune disorders and autoinflammatory syndromes. In the last decade, distinct cell and organ specific roles for NLRs have been identified however; their roles in cancer initiation, development and progression remain controversial. This review summarizes the emerging role of NLRs in cancer and their possible future as targets for cancer therapeutics.



Similar content being viewed by others
References
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Philip M, Rowley DA, Schreiber H (2004) Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol 14:433–439
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217
Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H (2012) The brain tumor microenvironment. Glia 60:502–514
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13:759–771
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Kasinski AL, Slack FJ (2011) MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 11:849–864
Lin W-W, Karin M (2007) A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Investig 117:1175
Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A (2009) Cellular and molecular pathways linking inflammation and cancer. Immunobiology 214:761–777
Ting JPY, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot J-P, Inohara N, MacKenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA (2008) The NLR gene family: a standard nomenclature. Immunity 28:285–287
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7:1243–1249
Ting JPY, Davis BK (2005) CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol 23:387–414
Ye Z, Ting JP-Y (2008) NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol 20:3–9
Kufer TA, Sansonetti PJ (2011) NLR functions beyond pathogen recognition. Nat Immunol 12:121–128
Zhong Y, Kinio A, Saleh M (2013) Functions of NOD-like receptors in human diseases. Front Immunol 4:333
Kent A, Blander JM (2014) Nod-like receptors: key molecular switches in the conundrum of cancer. Front Immunol 5:185
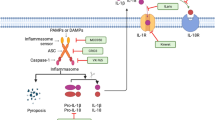
Schroder K, Tschopp J (2010) The Inflammasomes. Cell 140:821–832
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10:241–247
Srinivasula SM (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem 277:21119–21122
Keller M, Ruegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818–831
Feldmann J, Prieur A-M, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, Basile GDS (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 71:198–203
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 29:301–305
Conforti-Andreoni C, Ricciardi-Castagnoli P, Mortellaro A (2011) The inflammasomes in health and disease: from genetics to molecular mechanisms of autoinflammation and beyond. Cell Mol Immunol 8:135–145
Verma D, Lerm M, Blomgran-Julinder R, Eriksson P, Soderkvist P, Sarndahl E (2008) Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: relation to common inflammatory diseases? Arthritis Rheum 58:888–894
Paramel GV, Sirsjö A, Fransén K (2015) Role of genetic alterations in theNLRP3andCARD8Genes in health and disease. Mediators Inflamm 2015:1–10
De Nardo D, Latz E (2011) NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol 32:373–379
Ito S, Hara Y, Kubota T (2014) CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Res Ther 16:R52
Fransén K (2012) Role of NLRP3 and CARD8 in the regulation of TNF-α induced IL-1β release in vascular smooth muscle cells. Int J Mol Med 30:697–702
Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ, Mocarski ES, Dubyak GR (2015) Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem 290:20167–20184
Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Végran F, Ghiringhelli F (2015) The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol 16:859–870
Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2:541–546
Chassaing B, Aitken JD, Malleshappa M, Vijay‐Kumar M (2014) Dextran sulfate sodium (DSS)‐induced colitis in mice. Curr Protoc Immunol 15.25. 11–15.25. 14
Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP (2010) The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 207:1045–1056
Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD (2010) The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32:379–391
Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD (2010) IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 185:4912–4920
Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA 107:21635–21640
Oficjalska K, Raverdeau M, Aviello G, Wade SC, Hickey A, Sheehan KM, Corr SC, Kay EW, O’Neill LA, Mills KH (2015) Protective role for caspase-11 during acute experimental murine colitis. J Immunol 194:1252–1260
Verma D, Bivik C, Farahani E, Synnerstad I, Fredrikson M, Enerbäck C, Rosdahl I, Söderkvist P (2012) Inflammasome polymorphisms confer susceptibility to sporadic malignant melanoma. Pigment Cell Melanoma Res 25:506–513
Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, Zhong J, Meng G (2013) Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS ONE 8:e77955
Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, Zhao W, Huai W, Guo P, Han L (2013) Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest 94:52–62
Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini J-L, Schlemmer F, Tasdemir E, Uhl M, Génin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, André F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat Med 15:1170–1178
van Deventer HW, Burgents JE, Wu QP, Woodford RMT, Brickey WJ, Allen IC, McElvania-Tekippe E, Serody JS, Ting JPY (2010) The inflammasome component Nlrp3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res 70:10161–10169
Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, Voronov E (2013) The role of IL-1 in the early tumor cell-induced angiogenic response. J Immunol 190:3500–3509
Tarassishin L, Lim J, Weatherly DB, Angeletti RH, Lee SC (2014) Interleukin-1-induced changes in the glioblastoma secretome suggest its role in tumor progression. J Proteom 99:152–168
Tarassishin L, Casper D, Lee SC (2014) Aberrant expression of interleukin-1β and inflammasome activation in human malignant gliomas. PLoS ONE 9:e103432
Fathima K, Hurmath P, Ramaswamy DN (2014) Nandakumar, IL-1β microenvironment promotes proliferation, migration, and invasion of human glioma cells. Cell Biol Int 38:1415–1422
Poyet JL (2001) Identification of Ipaf, a human caspase-1-activating protein related to apaf-1. J Biol Chem 276:28309–28313
Hu Z, Yan C, Liu P, Huang Z, Ma R, Zhang C, Wang R, Zhang Y, Martinon F, Miao D (2013) Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341:172–175
Mayor A, Martinon F, De Smedt T, Pétrilli V, Tschopp J (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol 8:497–503
Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Kömüves L, Cupp JE, Arnott D, Monack D, Dixit VM (2012) Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490:539–542
Lage SL, Longo C, Branco LM, da Costa TsB, Buzzo CdL, Bortoluci KR (2014) Emerging concepts about NAIP/NLRC4 inflammasomes. Front Immunol 5:309
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11:1136–1142
Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, Shao F (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600
Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nuñez G (2007) Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in shigella-infected macrophages. PLoS Pathog 3:e111
Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM (2010) Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207:1745–1755
Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE (2014) Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci 111:7403–7408
Nordlander S, Pott J, Maloy KJ (2013) NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol 7(4):775–785
Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci 107:21635–21640
Roy N, Mahadevan MS, McLean M, Shutter G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, Salih M, Aubry H, Tamai K, Guan X, Ioannou P, Crawford TO, de Jong PJ, Surh L, Ikeda J-E, Korneluk RG, MacKenzie A (1995) The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80:167–178
Watihayati MS, Fatemeh H, Marini M, Atif AB, Zahiruddin WM, Sasongko TH, Tang TH, Zabidi-Hussin Z, Nishio H, Zilfalil BA (2009) Combination of SMN2 copy number and NAIP deletion predicts disease severity in spinal muscular atrophy. Brain Dev 31:42–45
Maier JKX, Balabanian S, Coffill CR, Stewart A, Pelletier L, Franks DJ, Gendron NH, MacKenzie AE (2007) Distribution of neuronal apoptosis inhibitory protein in human tissues. J Histochem Cytochem 55:911–923
Listen P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J-E, Mackenzie A, Korneluk RG (1996) Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349–353
Maier JK, Lahoua Z, Gendron NH, Fetni R, Johnston A, Davoodi J, Rasper D, Roy S, Slack RS, Nicholson DW (2002) The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J Neurosci 22:2035–2043
Davoodi J, Ghahremani M-H, Es-haghi A, Mohammad-gholi A, MacKenzie A (2010) Neuronal apoptosis inhibitory protein, NAIP, is an inhibitor of procaspase-9. Int J Biochem Cell Biol 42:958–964
Karimpour S, Davoodi J, Ghahremani M-H (2011) Integrity of ATP binding site is essential for effective inhibition of the intrinsic apoptosis pathway by NAIP. Biochem Biophys Res Commun 407:158–162
Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, N’Guessan PD, Gunther S, Schmeck B, Hippenstiel S, Flieger A, Suttorp N, Opitz B (2008) NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol 180:6808–6815
Yang J, Zhao Y, Shi J, Shao F (2013) Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci 110:14408–14413
Mercer EA, Korhonen L, Skoglösa Y, Olsson P-A, Kukkonen JP, Lindholm D (2000) NAIP interacts with hippocalcin and protects neurons against calcium-induced cell death through caspase-3-dependent and -independent pathways. EMBO J 19:3597–3607
Hutchison JS, Derrane RE, Johnston DL, Gendron N, Barnes D, Fliss H, King WJ, Rasquinha I, MacManus J, Robertson GS, MacKenzie AE (2001) Neuronal apoptosis inhibitory protein expression after traumatic brain injury in the mouse. J Neurotrauma 18:1333–1347
Martinon F, Tschopp J (2006) Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 14:10–22
Bai L, Wang S (2014) Targeting apoptosis pathways for new cancer therapeutics. Annu Rev Med 65:139–155
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348
Chen Y-K, Huse S-S, Lin L-M (2010) Expression of inhibitor of apoptosis family proteins in human oral squamous cell carcinogenesis. Head Neck 33:985–998
Choi J, Hwang YK, Choi YJ, Yoo KE, Kim JH, Nam SJ, Yang JH, Lee SJ, Yoo KH, Sung KW, Koo HH, Im Y-H (2007) Neuronal apoptosis inhibitory protein is overexpressed in patients with unfavorable prognostic factors in breast cancer. J Korean Med Sci 22:S17
Mazrouei S, Ziaei A, Tanhaee A, Keyhanian K, Esmaeili M, Baradaran A, Salehi M (2012) Apoptosis inhibition or inflammation: the role of NAIP protein expression in Hodgkin and non-Hodgkin lymphomas compared to non-neoplastic lymph node. J Inflamm 9:4
Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, Kallioniemi O-P, Shabaik A, Vitiello A, Peehl D (2003) Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res 9:4914–4925
Chiu HHL, Yong TMK, Wang J, Wang Y, Vessella RL, Ueda T, Wang Y-Z, Sadar MD (2010) Induction of neuronal apoptosis inhibitory protein expression in response to androgen deprivation in prostate cancer. Cancer Lett 292:176–185
Endo T, Abe S, Seidlar HBK, Nagaoka S, Takemura T, Utsuyama M, Kitagawa M, Hirokawa K (2004) Expression of IAP family proteins in colon cancers from patients with different age groups. Cancer Immunol Immunother 53:770–776
Nemoto T, Kitagawa M, Hasegawa M, Ikeda S, Akashi T, Takizawa T, Hirokawa K, Koike M (2004) Expression of IAP family proteins in esophageal cancer. Exp Mol Pathol 76:253–259
Grenier JM, Wang L, Manji GA, Huang W-J, Al-Garawi A, Kelly R, Carlson A, Merriam S, Lora JM, Briskin M, DiStefano PS, Bertin J (2002) Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-κB and caspase-1. FEBS Lett 530:73–78
Chen GY (2014) Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur J Immunol 44:321–327
Anand PK, Malireddi RKS, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti T-D (2012) NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488:389–393
Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA (2011) NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145(2011):745–757
Chen GY, Liu M, Wang F, Bertin J, Nunez G (2011) A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol 186:7187–7194
Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M (2011) Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci 108:9601–9606
Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, Flavell RA (2013) Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci 110:9862–9867
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10:417–426
Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M (2008) A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA 105:7803–7808
Ewald SE, Chavarria-Smith J, Boothroyd JC (2014) NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun 82:460–468
Chavarría-Smith J, Vance RE (2013) Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog 9(6):e1003452
Chavarría-Smith J, Vance RE (2015) The NLRP1 inflammasomes. Immunol Rev 265:22–34
Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, Bertin JJ, Gough PJ (2012) Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem 287:25030–25037
Bruey J-M, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A (2007) Bcl-2 and Bcl-X L regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 129:45–56
Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S-I, Reed JC, Ting JP, Damania B (2011) Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331:330–334
Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC (2007) Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25:713–724
Mille F, Thibert C, Fombonne J, Rama N, Guix C, Hayashi H, Corset V, Reed JC, Mehlen P (2009) The Patched dependence receptor triggers apoptosis through a DRAL–caspase-9 complex. Nat Cell Biol 11:739–746
Pontillo A, Catamo E, Arosio B, Mari D, Crovella S (2012) NALP1/NLRP1 genetic variants are associated with Alzheimer disease. Alzheimer Dis Assoc Disord 26:277–281
Sui J, Li H, Fang Y, Liu Y, Li M, Zhong B, Yang F, Zou Q, Wu Y (2012) NLRP1 gene polymorphism influences gene transcription and is a risk factor for rheumatoid arthritis in Han Chinese. Arthritis Rheum 64:647–654
Tan M, Tan L, Jiang T, Zhu X, Wang H, Jia C, Yu J (2014) Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis 5:e1382
de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW (2009) Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 29(7):1251–1261
Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ (2012) NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37:1009–1023
Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP, Koller BH (2012) NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J Immunol 189:2006–2016
Girardelli M, Maestri I, Rinaldi RR, Tognon M, Boldorini R, Bovenzi M, Crovella S, Comar M (2012) NLRP1 polymorphisms in patients with asbestos-associated mesothelioma. Infect Agent Cancer 7:25
Williams TM, Leeth RA, Rothschild DE, Coutermarsh-Ott SL, McDaniel DK, Simmons AE, Heid B, Cecere TE, Allen IC (2015) The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J Immunol 194:3369–3380
DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, Meltzer PS, Trent JM (1997) Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15:453–457
Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518
Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA, Aderem A (2010) Cutting edge: cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol 185:818–821
Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ (2013) AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20:1149–1160
Morrone SR, Matyszewski M, Yu X, Delannoy M, Egelman EH, Sohn J (2015) Assembly-driven activation of the AIM2 foreign-dsDNA sensor provides a polymerization template for downstream ASC. Nat Commun 6:7827
Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11:395–402
Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K (2012) Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol 24:637–644
Sauer J-D, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA (2010) Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7:412–419
Fernandes-Alnemri T, Yu J-W, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11:385–393
Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T (2012) AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19:1709–1721
Crane DD, Bauler TJ, Wehrly TD, Bosio CM (2014) Mitochondrial ROS potentiates indirect activation of the AIM2 inflammasome. Front Microbiol 5:438
Man SM, Karki R, Malireddi RKS, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti T-D (2015) The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 16:467–475
Kalantari P, DeOliveira RB, Chan J, Corbett Y, Rathinam V, Stutz A, Latz E, Gazzinelli RT, Golenbock DT, Fitzgerald KA (2014) Dual Engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during Malaria. Cell Rep 6:196–210
Park E et al (2014) Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun 82:112–123
Duan X, Ponomareva L, Veeranki S, Panchanathan R, Dickerson E, Choubey D (2011) Differential Roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts. Mol Cancer Res 9(5):589–602
Zhao C, Gillette DD, Li X, Zhang Z, Wen H (2014) Nuclear Factor E2-related Factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J Biol Chem 289:17020–17029
Jin T, Perry A, Smith P, Jiang J, Xiao TS (2013) Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem 288:13225–13235
Wang L-J, Huang H-Y, Huang M-P, Liou W, Chang Y-T, Wu C-C, Ojcius DM, Chang Y-S (2014) The microtubule-associated protein EB1 links AIM2 inflammasomes with autophagy-dependent secretion. J Biol Chem 289:29322–29333
Hanamsagar R, Aldrich A, Kielian T (2014) Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem 129:704–711
Denes A, Coutts G, Lénárt N, Cruickshank SM, Pelegrin P, Skinner J, Rothwell N, Allan SM, Brough D (2015) AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci 112:4050–4055
Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M (2014) Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer 135:2387–2396
Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RKS, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti T-D (2015) Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell 162:45–58
Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Mühlbauer M, Chou W-C, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JPY (2015) Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med 21:906–913
Chen IF (2006) AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther 5:1–7
Patsos G, Germann A, Gebert J, Dihlmann S (2009) Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer
Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D (2013) AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res 11:1193–1202
Lee J, Li L, Gretz N, Gebert J, Dihlmann S (2012) Absent in Melanoma 2 (AIM2) is an important mediator of interferon-dependent and-independent HLA-DRA and HLA-DRB gene expression in colorectal cancers. Oncogene 31:1242–1253
Liu R, Truax A, Chen L, Hu P, Li Z, Chen J, Song C, Ting J (2015) Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget 6(32):33456–33469
Hasegawa M, Imamura R, Motani K, Nishiuchi T, Matsumoto N, Kinoshita T, Suda T (2009) Mechanism and repertoire of ASC-mediated gene expression. J Immunol 182:7655–7662
Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC (2003) Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol 171:6154–6163
Masumoto J, Dowds TA, Schaner P, Chen FF, Ogura Y, Li M, Zhu L, Katsuyama T, Sagara J, Taniguchi SI, Gumucio DL, Núñez G, Inohara N (2003) ASC is an activating adaptor for NF-B and caspase-8-dependent apoptosis. Biochem Biophys Res Commun 303:69–73
Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, Aaronson SA, Lee SW (2004) ASC is a Bax adaptor and regulates the p53–Bax mitochondrial apoptosis pathway. Nat Cell Biol 6:121–128
Sarkar A, Duncan M, Hart J, Hertlein E, Guttridge DC, Wewers MD (2006) ASC Directs NF-B activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol 176:4979–4986
McConnell BB, Vertino PM (2004) TMS1/ASC: the cancer connection. Apoptosis 9:5–18
Levine JJ, Stimson-Crider KM, Vertino PM (2003) Effects of methylation on expression of TMS1/ASC in human breast cancer cells. Oncogene 22:3475–3488
Yokoyama T, Sagara J, Guan X, Masumoto J, Takeoka M, Komiyama Y, Miyata K, Higuchi K (2003) S.i. Taniguchi, Methylation of ASC/TMS1, a proapoptotic gene responsible for activating procaspase-1, in human colorectal cancer. Cancer Lett 202:101–108
Terasawa K (2004) Epigenetic Inactivation of TMS1/ASC in Ovarian Cancer. Clin Cancer Res 10:2000–2006
Machida EO (2006) Hypermethylation of ASC/TMS1 is a sputum marker for late-stage lung cancer. Cancer Res 66:6210–6218
Das PM, Ramachandran K, Vanwert J, Ferdinand L, Gopisetty G, Reis IM, Singal R (2006) Methylation mediated silencing of TMS1/ASC gene in prostate cancer. Mol Cancer 5:28
Stone AR, Bobo W, Brat DJ, Devi NS, Van Meir EG, Vertino PM (2004) Aberrant methylation and down-regulation of TMS1/ASC in human glioblastoma. Am J Pathol 165:1151–1161
Martinez R, Schackert G, Esteller M (2006) Hypermethylation of the proapoptotic gene TMS1/ASC: prognostic importance in glioblastoma multiforme. J Neurooncol 82:133–139
Zhang C, Li H, Zhou G, Zhang Q, Zhang T, Li J, Zhang J, Hou J, Liew CT, Yin D (2007) Transcriptional silencing of the TMS1/ASC tumour suppressor gene by an epigenetic mechanism in hepatocellular carcinoma cells. J Pathol 212:134–142
Liu W, Luo Y, Dunn JH, Norris DA, Dinarello CA, Fujita M (2012) Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J Investig Dermatol 133:518–527
Knight ERW, Patel EY, Flowers CA, Crowther AJ, Ting JP, Miller CR, Gershon TR, Deshmukh M (2014) ASC deficiency suppresses proliferation and prevents medulloblastoma incidence. Oncogene 34:394–402
Ting JPY, Duncan JA, Lei Y (2010) How the noninflammasome NLRs function in the innate immune system. Science 327:286–290
Allen IC (2014) Non-inflammasome forming NLRs in inflammation and tumorigenesis. Front Immunol 5:169
Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J (2002) PYPAF7, a Novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem 277:29874–29880
Pinheiro AS, Eibl C, Ekman-Vural Z, Schwarzenbacher R, Peti W (2011) The NLRP12 pyrin domain: structure, dynamics, and functional insights. J Mol Biol 413:790–803
Williams KL, Taxman DJ, Linhoff MW, Reed W, Ting JPY (2003) Cutting Edge: monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J Immunol 170:5354–5358
Williams KL (2005) The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor-, and mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem 280:39914–39924
Ye Z, Lich JD, Moore CB, Duncan JA, Williams KL, Ting JPY (2007) ATP binding by monarch-1/NLRP12 is critical for its inhibitory function. Mol Cell Biol 28:1841–1850
Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JPY (2007) Cutting edge: monarch-1 suppresses non-canonical NF-B activation and p52-dependent chemokine expression in monocytes. J Immunol 178:1256–1260
Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RMT, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JPY (2012) NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36:742–754
Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD (2013) Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proc Natl Acad Sci 111:385–390
Zaki MH, Vogel P, Malireddi RKS, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti T-D (2011) The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20:649–660
Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JPY (2008) NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451:573–577
Xiao TS, Ting JPY (2012) NLRX1 has a tail to tell. Immunity 36:311–312
Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, Ranjan P, Monroe KM, Pickles RJ, Sambhara S, Ting JPY (2011) NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34:854–865
Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ, Wang R-F (2011) NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34:843–853
Tattoli I, Carneiro LA, Jéhanno M, Magalhaes JG, Shu Y, Philpott DJ, Arnoult D, Girardin SE (2008) NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep 9:293–300
Hong M, Yoon S-I, Wilson IA (2012) Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity 36:337–347
Soares F, Tattoli I, Wortzman ME, Arnoult D, Philpott DJ, Girardin SE (2012) NLRX1 does not inhibit MAVS-dependent antiviral signalling. Innate Immun 19:438–448
Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, Swanson KV, Wen K-W, Damania B, Moore CB, Giguère PM, Siderovski DP, Hiscott J, Razani B, Semenkovich CF, Chen X, Ting JPY (2012) The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36:933–946
Soares F, Tattoli I, Rahman MA, Robertson SJ, Belcheva A, Liu D, Streutker C, Winer S, Winer DA, Martin A, Philpott DJ, Arnoult D, Girardin SE (2014) The mitochondrial protein NLRX1 controls the balance between extrinsic and intrinsic apoptosis. J Biol Chem 289:19317–19330
Singh K, Poteryakhina A, Zheltukhin A, Bhatelia K, Prajapati P, Sripada L, Tomar D, Singh R, Singh AK, Chumakov PM, Singh R (1853) NLRX1 acts as tumor suppressor by regulating TNF-α induced apoptosis and metabolism in cancer cells. Biochim Biophys Acta 2015:1073–1086
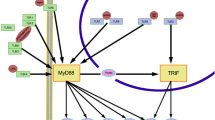
Correa RG, Milutinovic S, Reed JC (2012) Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep 32:597–608
Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE (2013) NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14:9–23
Bertrand MJM, Doiron K, Labbé K, Korneluk RG, Barker PA, Saleh M (2009) Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30:789–801
Lecine P, Esmiol S, Metais JY, Nicoletti C, Nourry C, McDonald C, Nunez G, Hugot JP, Borg JP, Ollendorff V (2007) The NOD2-RICK complex signals from the plasma membrane. J Biol Chem 282:15197–15207
Kim Y-G, Park J-H, Shaw MH, Franchi L, Inohara N, Núñez G (2008) The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to toll-like receptor ligands. Immunity 28:246–257
Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S (2003) An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 4:702–707
Travassos LH (2005) Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem 280:36714–36718
Grubman A, Kaparakis M, Viala J, Allison C, Badea L, Karrar A, Boneca IG, Le Bourhis L, Reeve S, Smith IA, Hartland EL, Philpott DJ, Ferrero RL (2010) The innate immune molecule, NOD1, regulates direct killing ofHelicobacter pyloriby antimicrobial peptides. Cell Microbiol 12:626–639
Silva GK, Gutierrez FRS, Guedes PMM, Horta CV, Cunha LD, Mineo TWP, Santiago-Silva J, Kobayashi KS, Flavell RA, Silva JS, Zamboni DS (2009) Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against trypanosoma cruzi infection. J Immunol 184:1148–1152
Tsuji Y, Watanabe T, Kudo M, Arai H, Strober W, Chiba T (2012) Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity 37:326–338
Strober W, Murray PJ, Kitani A, Watanabe T (2005) Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 6:9–20
Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA (2012) Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J Biol Chem 287:23057–23067
von Kampen O, Lipinski S, Till A, Martin SJ, Nietfeld W, Lehrach H, Schreiber S, Rosenstiel P (2010) Caspase recruitment domain-containing protein 8 (CARD8) negatively regulates NOD2-mediated signaling. J Biol Chem 285:19921–19926
Mohanan V, Grimes CL (2014) The molecular chaperone HSP70 binds to and stabilizes NOD2, an important protein involved in crohn disease. J Biol Chem 289:18987–18998
Kanneganti T-D, Lamkanfi M, Núñez G (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27:549–559
Chen GY, Shaw MH, Redondo G, Núñez G (2008) The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res 68:10060–10067
Wang X, Jiang W, Duan N, Qian Y, Zhou Q, Ye P, Jiang H, Bai Y, Zhang W, Wang W (2014) NOD1, RIP2 and Caspase12 are potentially novel biomarkers for oral squamous cell carcinoma development and progression. Int J Clin Exp Pathol 7:1677
Zaki MH (2013) NOD-like receptors in colon tumorigenesis. Immunogastroenterology 2:90
Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A (2013) NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Investig 123:700
Kurzawski G, Suchy J, Kładny J, Grabowska E, Mierzejewski M, Jakubowska A, Dȩbniak T, Cybulski C, Kowalska E, Szych Z (2004) The NOD2 3020insC mutation and the risk of colorectal cancer. Cancer Res 64:1604–1606
Kutikhin AG (2011) Role of NOD1/CARD4 and NOD2/CARD15 gene polymorphisms in cancer etiology. Hum Immunol 72:955–968
Gabay C, Lamacchia C, Palmer G (2010) IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 6:232–241
Dhimolea E (2011) Interleukin-1beta; inhibitors for the treatment of cryopyrin-associated periodic syndrome. Appl Clin Genet 4:21–27
Zitvogel L, Kepp O, Galluzzi L, Kroemer G (2012) Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol 13:343–351
Shen H, Sun T, Ferrari M (2012) Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther 19:367–373
Friedman A (2006) Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res 66:2314–2319
Cattaneo R, Miest T, Shashkova EV, Barry MA (2008) Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Micro 6:529–540
Hegi ME, Rajakannu P, Weller M (2012) Epidermal growth factor receptor. Curr Opin Neurol 25:774–779
Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK (2012) Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res 72:402–407
Luo JL (2005) IKK/NF-B signaling: balancing life and death: a new approach to cancer therapy. J Clin Investig 115:2625–2632
López-Castejón G, Pelegrín P (2012) Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin Investig Drugs 21:995–1007
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P (2012) Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 12:860–875
Acknowledgments
SJ’s laboratory is funded by grants from the Department of Science and Technology (Young scientist scheme, SB/YS/LS-282/2013) and Board of Research in Nuclear Sciences (2013/36/72-BRNS/2415), Government of India. The software application Science Slides (VisiScience) was used to generate parts of Figs. 1, 2 and 3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, N., Jha, S. NLR-regulated pathways in cancer: opportunities and obstacles for therapeutic interventions. Cell. Mol. Life Sci. 73, 1741–1764 (2016). https://doi.org/10.1007/s00018-015-2123-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2123-8