Abstract
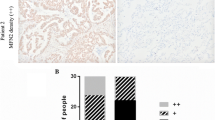
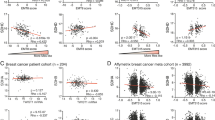
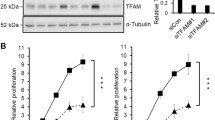
Mitochondrial dysfunction and epithelial-to-mesenchymal transition (EMT) play important roles in cancer development and metastasis. However, very little is known about the connection between mitochondrial dysfunction and EMT. Tu translation elongation factor, mitochondrial (TUFM), a key factor in the translational expression of mitochondrial DNA, plays an important role in the control of mitochondrial function. Here, we show that TUFM is downregulated in human cancer tissues. TUFM expression level was positively correlated with that of E-cadherin and decreased significantly during the progression of human lung cancer. TUFM knockdown induced EMT, reduced mitochondrial respiratory chain activity, and increased glycolytic function and the production of reactive oxygen species (ROS). Mechanistically, TUFM knockdown activated AMPK and phosphorylated GSK3β and increased the nuclear accumulation of β-catenin, leading to the induction of EMT and increased migration and metastasis of A549 lung cancer cells. Although TUFM knockdown also induced EMT of MCF7 breast cancer cells, the underlying mechanism appeared somewhat different from that in lung cancer cells. Our work identifies TUFM as a novel regulator of EMT and suggests a molecular link between mitochondrial dysfunction and EMT induction.








Similar content being viewed by others
Abbreviations
- EMT:
-
Epithelial-to-mesenchymal transition
- TUFM:
-
Tu translation elongation factor, mitochondrial
- GLUT1:
-
Glucose transporter 1
- GLUT4:
-
Glucose transporter 4
- LDHA:
-
Lactate dehydrogenase A
- ECAR:
-
Extracellular acidification rate
- 2-DG:
-
2-Deoxyglucose
- AMPK:
-
AMP-activated protein kinase
- GSK3β:
-
Glycogen synthase kinase 3β
- ACC:
-
Acetyl-CoA carboxylase
- AXIN2:
-
Axis inhibition protein 2
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- CTCs:
-
Circulating tumor cells
References
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283(5407):1482–1488
Ohsawa S, Sato Y, Enomoto M, Nakamura M, Betsumiya A, Igaki T (2012) Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature 490(7421):547–551. doi:10.1038/nature11452
Modica-Napolitano JS, Singh KK (2004) Mitochondrial dysfunction in cancer. Mitochondrion 4(5–6):755–762
Brandon M, Baldi P, Wallace DC (2006) Mitochondrial mutations in cancer. Oncogene 25(34):4647–4662
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial–mesenchymal transitions in development and disease. Cell 139(5):871–890. doi:10.1016/j.cell.2009.11.007
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196. doi:10.1038/nrm3758
Lu X, Kang Y (2010) Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res 16(24):5928–5935. doi:10.1158/1078-0432.CCR-10-1360
Giannoni E, Parri M, Chiarugi P (2012) EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal 16(11):1248–1263. doi:10.1089/ars.2011.4280
Guha M, Srinivasan S, Ruthel G, Kashina AK, Carstens RP, Mendoza A et al (2014) Mitochondrial retrograde signaling induces epithelial–mesenchymal transition and generates breast cancer stem cells. Oncogene 33(45):5238–5250. doi:10.1038/onc.2013.467
Favre C, Zhdanov A, Leahy M, Papkovsky D, O’Connor R (2010) Mitochondrial pyrimidine nucleotide carrier (PNC1) regulates mitochondrial biogenesis and the invasive phenotype of cancer cells. Oncogene 29(27):3964–3976. doi:10.1038/onc.2010.146
Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G (2005) TGFβ1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 24(11):1895–1903
Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI et al (2012) Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res 72(14):3607–3617. doi:10.1158/0008-5472
Yuan Y, Chen Y, Zhang P, Huang S, Zhu C, Ding G et al (2012) Mitochondrial dysfunction accounts for aldosterone-induced epithelial-to-mesenchymal transition of renal proximal tubular epithelial cells. Free Radic Biol Med 53(1):30–43. doi:10.1016/j.freeradbiomed.2012.03.015
Christian BE, Spremulli LL (2012) Mechanism of protein biosynthesis in mammalian mitochondria. Biochim Biophys Acta. Biochim Biophys Acta 1819(9–10):1035–1054. doi:10.1016/j.bbagrm.2011.11.009
Zhang KH, Tian HY, Gao X, Lei WW, Hu Y, Wang DM et al (2009) Ferritin heavy chain-mediated iron homeostasis and subsequent increased reactive oxygen species production are essential for epithelial–mesenchymal transition. Cancer Res 69(13):5340–5348. doi:10.1158/0008-5472.CAN-09-0112
Hu Y, He K, Wang D, Yuan X, Liu Y, Ji H et al (2013) TMEPAI regulates EMT in lung cancer cells by modulating the ROS and IRS-1 signaling pathways. Carcinogenesis 34(8):1764–1772. doi:10.1093/carcin/bgt132
Shi J, Wang DM, Wang CM, Hu Y, Liu AH, Zhang YL et al (2009) Insulin receptor substrate-1 suppresses transforming growth factor-β1-mediated epithelial–mesenchymal transition. Cancer Res 69(18):7180–7187. doi:10.1158/0008-5472.CAN-08-4470
Tiscornia G, Singer O, Verma IM (2006) Production and purification of lentiviral vectors. Nat Protoc 1(1):241–245
Wang Z, Fan M, Candas D, Zhang TQ, Qin L, Eldridge A et al (2014) Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev Cell 29(2):217–232. doi:10.1016/j.devcel.2014.03.012
Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z et al (2011) Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 20(5):674–688. doi:10.1016/j.ccr.2011.10.015
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA et al (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278(10):8516–8525
Park WH, Han YW, Kim SH, Kim SZ (2007) An ROS generator, antimycin A, inhibits the growth of HeLa cells via apoptosis. J Cell Biochem 102(1):98–109
Qu J, Miao H, Ma Y, Guo F, Deng J, Wei X et al (2014) Loss of Abhd5 promotes colorectal tumor development and progression by inducing aerobic glycolysis and epithelial–mesenchymal transition. Cell Rep 9(5):1798–1811. doi:10.1016/j.celrep.2014.11.016
Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1(1):15–25
Hardie DG, Salt IP, Hawley SA, Davies SP (1999) AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J 338(Pt3):717–722
Wang X, Pan X, Song J (2010) AMP-activated protein kinase is required for induction of apoptosis and epithelial-to-mesenchymal transition. Cell Signal 22(11):1790–1797. doi:10.1016/j.cellsig.2010.07.008
Landis J, Shaw LM (2014) Insulin receptor substrate 2-mediated phosphatidylinositol 3-kinase signaling selectively inhibits glycogen synthase kinase 3β to regulate aerobic glycolysis. J Biol Chem 289(26):18603–18613. doi:10.1074/jbc.M114.564070
Gonzalez DM, Medici D (2014) Signaling mechanisms of the epithelial–mesenchymal transition. Sci Signal 7(344):re8. doi:10.1126/scisignal.2005189
Kim K, Lu Z, Hay ED (2002) Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int 26(5):463–476
Moro L, Arbini AA, Yao JL, di Sant’Agnese PA, Marra E, Greco M (2009) Mitochondrial DNA depletion in prostate epithelial cells promotes anoikis resistance and invasion through activation of PI3 K/Akt2. Cell Death Differ 16(4):571–583. doi:10.1038/cdd.2008.178
Kamarajugadda S, Stemboroski Cai Q, Simpson NE, Nayak S, Tan M et al (2012) Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol 32(10):1893–1907. doi:10.1128/MCB.06248-11
Burstyn-Cohen T, Kalcheim C (2002) Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Dev Cell 3(3):383–395
Yang Y, Pan X, Lei W, Wang J, Song J (2006) Transforming growth factor-β1 induces epithelial-to-mesenchymal transition and apoptosis via a cell cycle-dependent mechanism. Oncogene 25(55):7235–7244
Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18(10):1131–1143
Urasaki Y, Heath L, Xu CW (2012) Coupling of glucose deprivation with impaired histone H2B monoubiquitination in tumors. PLoS ONE 7(5):e36775. doi:10.1371/journal.pone.0036775
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H et al (2009) Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res 69(11):4918–4925. doi:10.1158/0008-5472.CAN-08-4806
Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B et al (2014) Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508(7494):108–112. doi:10.1038/nature13110
Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L et al (2013) Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155(7):1624–1638. doi:10.1016/j.cell.2013.11.037
Johnson KR, Zheng QY, Bykhovskaya Y, Spirina O, Fischel-Ghodsian N (2001) A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nat Genet 27(2):191–194
Roubertoux PL, Sluyter F, Carlier M, Marcet B, Maarouf-Veray F, Cherif C et al (2003) Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet 35(1):65–69
Jeon SM, Chandel NS, Hay N (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485(7400):661–665. doi:10.1038/nature11066
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z (2012) ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14(12):1295–1304. doi:10.1038/ncb2629
Shin SM, Cho IJ, Kim SG (2009) Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3β inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol 76(4):884–895. doi:10.1124/mol.109.058479
Choi SH, Kim YW, Kim SG (2010) AMPK-mediated GSK3β inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol 79(9):1352–1362. doi:10.1016/j.bcp.2009.12.011
BE Fitzwalter, Thorburn A (2015) Recent insights into cell death and autophagy. FEBS J. Sep 14. doi: 10.1111/febs.13515 (Epub ahead of print)
Cicchini M, Karantza V, Xia B (2015) Molecular pathways: autophagy in cancer—a matter of timing and context. Clin Cancer Res 21(3):498–504. doi:10.1158/1078-0432.CCR-13-2438
Liang J, Xu ZX, Ding Z, Lu Y, Yu Q, Werle KD et al (2015) Myristoylation confers noncanonical AMPK functions in autophagy selectivity and mitochondrial surveillance. Nat Commun 6:7926. doi:10.1038/ncomms8926
Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y et al (2013) Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition. Carcinogenesis 34(6):1343–1351. doi:10.1093/carcin/bgt063
Acknowledgments
We thank Drs. Yunneng Tang, Guangwen Shu, and other members of the laboratory for their critical comments and insightful discussions. This work was supported by the National Science Foundation of China (81472603) and the Chinese Ministry of Science and Technology (2011CB966200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Kai He and Xiaojie Guo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, K., Guo, X., Liu, Y. et al. TUFM downregulation induces epithelial–mesenchymal transition and invasion in lung cancer cells via a mechanism involving AMPK-GSK3β signaling. Cell. Mol. Life Sci. 73, 2105–2121 (2016). https://doi.org/10.1007/s00018-015-2122-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2122-9