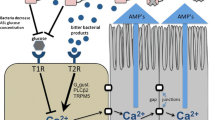
Abstract
Taste receptors were first identified on the tongue, where they initiate a signaling pathway that communicates information to the brain about the nutrient content or potential toxicity of ingested foods. However, recent research has shown that taste receptors are also expressed in a myriad of other tissues, from the airway and gastrointestinal epithelia to the pancreas and brain. The functions of many of these extraoral taste receptors remain unknown, but emerging evidence suggests that bitter and sweet taste receptors in the airway are important sentinels of innate immunity. This review discusses taste receptor signaling, focusing on the G-protein–coupled receptors that detect bitter, sweet, and savory tastes, followed by an overview of extraoral taste receptors and in-depth discussion of studies demonstrating the roles of taste receptors in airway innate immunity. Future research on extraoral taste receptors has significant potential for identification of novel immune mechanisms and insights into host-pathogen interactions.





Similar content being viewed by others
Abbreviations
- ACh:
-
Acetylcholine
- AHL:
-
Acyl-homoserine lactone
- AMP:
-
Antimicrobial peptide
- ASL:
-
Airway surface liquid
- ATP:
-
Adenosine trisphophate
- C4HSL:
-
N-butyryl-L-homoserine lactone
- C12HSL:
-
N-3-oxo-dodecanoyl-L-homoserine lactone
- CALHM1:
-
Calcium homeostasis modulator isoform 1
- cAMP:
-
Cyclic adenosine monophosphate
- CGRP:
-
Calcitonin gene-related peptide
- COPD:
-
Chronic obstructive pulmonary disease
- CRS:
-
Chronic rhinosinusitis
- CSF:
-
Cerebrospinal fluid
- ENaC:
-
Epithelial sodium channel
- ER:
-
Endoplasmic reticulum
- GPCR:
-
G-protein–coupled receptor
- IP3 :
-
Inositol 1,4,5-trisphosphate
- IP3R3:
-
Inositol trisphosphate receptor isoform 3
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PDE:
-
Phosphodiesterase
- PKA:
-
cAMP-dependent protein kinase A
- PKG:
-
cGMP-dependent protein kinase G
- PLCβ2:
-
Phospholipase C isoform β2
- PROP:
-
Propylthiouracil
- PTC:
-
Phenylthiocarbamide, also known as phenylthiourea
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SCC:
-
Solitary chemosensory cell
- T1R:
-
Taste receptor family 1 protein isoform
- T2R:
-
Taste receptor family 2 protein isoform
- TAS1R :
-
Taste receptor family 1 gene
- TAS2R :
-
Taste receptor family 2 gene
- TLR:
-
Toll-like receptor
- TRPM5:
-
Transient receptor potential cation channel subfamily M isoform
References
Blalock JE (2005) The immune system as the sixth sense. J Intern Med 257(2):126–138. doi:10.1111/j.1365-2796.2004.01441.x
Blalock JE, Smith EM (2007) Conceptual development of the immune system as a sixth sense. Brain Behav Immun 21(1):23–33. doi:10.1016/j.bbi.2006.09.004
Bedford FL (2011) The missing sense modality: the immune system. Perception 40(10):1265–1267
Deckmann K, Filipski K, Krasteva-Christ G, Fronius M, Althaus M, Rafiq A, Papadakis T, Renno L, Jurastow I, Wessels L, Wolff M, Schutz B, Weihe E, Chubanov V, Gudermann T, Klein J, Bschleipfer T, Kummer W (2014) Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci USA 111(22):8287–8292. doi:10.1073/pnas.1402436111
Margolskee RF (2005) Teaching resources. Sensory systems: taste perception. Sci STKE 290:tr20. doi:10.1126/stke.2902005tr20
Margolskee RF (1993) The molecular biology of taste transduction. BioEssays 15(10):645–650. doi:10.1002/bies.950151003
Beauchamp GK, Mennella JA (2011) Flavor perception in human infants: development and functional significance. Digestion 83(Suppl 1):1–6. doi:10.1159/000323397
Wise PM, Wolf M, Thom SR, Bryant B (2013) The influence of bubbles on the perception carbonation bite. PLoS One 8(8):e71488. doi:10.1371/journal.pone.0071488
Viana F (2011) Chemosensory properties of the trigeminal system. ACS Chem Neurosci 2(1):38–50. doi:10.1021/cn100102c
Breslin PA, Huang L (2006) Human taste: peripheral anatomy, taste transduction, and coding. Adv Otorhinolaryngol 63:152–190. doi:10.1159/000093760
Kinnamon SC (2012) Taste receptor signalling—from tongues to lungs. Acta Physiol (Oxf) 204(2):158–168. doi:10.1111/j.1748-1716.2011.02308.x
Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman R (2006) The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Des 12(35):4591–4600
Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF (2001) Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28(1):58–63. doi:10.1038/88270
Ozeck M, Brust P, Xu H, Servant G (2004) Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol 489(3):139–149. doi:10.1016/j.ejphar.2004.03.004
Margolskee RF (2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277(1):1–4. doi:10.1074/jbc.R100054200
Lin W, Finger TE, Rossier BC, Kinnamon SC (1999) Epithelial Na + channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405(3):406–420
Kretz O, Barbry P, Bock R, Lindemann B (1999) Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47(1):51–64
Eylam S, Spector AC (2003) Oral amiloride treatment decreases taste sensitivity to sodium salts in C57BL/6 J and DBA/2 J mice. Chem Senses 28(5):447–458
Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ (2003) Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron 39(1):133–146
Ben-Shahar Y (2011) Sensory functions for degenerin/epithelial sodium channels (DEG/ENaC). Adv Genet 76:1–26. doi:10.1016/B978-0-12-386481-9.00001-8
He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S (2004) Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24(35):7674–7680. doi:10.1523/JNEUROSCI.2441-04.2004
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002) Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99(7):4692–4696. doi:10.1073/pnas.072090199
Rong M, He W, Yasumatsu K, Kokrashvili Z, Perez CA, Mosinger B, Ninomiya Y, Margolskee RF, Damak S (2005) Signal transduction of umami taste: insights from knockout mice. Chem Senses 30(Suppl 1):i33–i34. doi:10.1093/chemse/bjh099
Beauchamp GK (2009) Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 90(3):723S–727S. doi:10.3945/ajcn.2009.27462E
Benarroch EE (2014) Acid-sensing cation channels: structure, function, and pathophysiologic implications. Neurology 82(7):628–635. doi:10.1212/WNL.0000000000000134
Holzer P (2009) Acid-sensitive ion channels and receptors. Handb Exp Pharmacol 194:283–332. doi:10.1007/978-3-540-79090-7_9
Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S (2006) Acid-sensing ion channels in taste buds. Arch Histol Cytol 69(4):227–231
Kinnamon SC, Margolskee RF (1996) Mechanisms of taste transduction. Curr Opin Neurobiol 6(4):506–513
Wong GT, Gannon KS, Margolskee RF (1996) Transduction of bitter and sweet taste by gustducin. Nature 381(6585):796–800. doi:10.1038/381796a0
Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS (2000) A novel family of mammalian taste receptors. Cell 100(6):693–702
Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ (2000) T2Rs function as bitter taste receptors. Cell 100(6):703–711
Scott K (2004) The sweet and the bitter of mammalian taste. Curr Opin Neurobiol 14(4):423–427. doi:10.1016/j.conb.2004.06.003
Iwata S, Yoshida R, Ninomiya Y (2014) Taste transductions in taste receptor cells: basic tastes and moreover. Curr Pharm Des 20(16):2684–2692
Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112(3):293–301
Kojima I, Nakagawa Y, Ohtsu Y, Medina A, Nagasawa M (2014) Sweet taste-sensing receptors expressed in pancreatic beta-cells: sweet molecules act as biased agonists. Endocrinol Metab (Seoul) 29(1):12–19. doi:10.3803/EnM.2014.29.1.12
Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I (2013) Multimodal function of the sweet taste receptor expressed in pancreatic beta-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J 60(10):1191–1206
Medina A, Nakagawa Y, Ma J, Li L, Hamano K, Akimoto T, Ninomiya Y, Kojima I (2014) Expression of the glucose-sensing receptor T1R3 in pancreatic islet: changes in the expression levels in various nutritional and metabolic states. Endocr J 61(8):797–805
Nakagawa Y, Ohtsu Y, Nagasawa M, Shibata H, Kojima I (2014) Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J 61(2):119–131
Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, Kurose H, Kojima I, Shibata H (2013) A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS One 8(1):e54500. doi:10.1371/journal.pone.0054500
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106(3):381–390
Tordoff MG, Shao H, Alarcon LK, Margolskee RF, Mosinger B, Bachmanov AA, Reed DR, McCaughey S (2008) Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics 34(3):338–348. doi:10.1152/physiolgenomics.90200.2008
Kuhn C, Bufe B, Batram C, Meyerhof W (2010) Oligomerization of TAS2R bitter taste receptors. Chem Senses 35(5):395–406. doi:10.1093/chemse/bjq027
Kuhn C, Meyerhof W (2013) Oligomerization of sweet and bitter taste receptors. Methods Cell Biol 117:229–242. doi:10.1016/B978-0-12-408143-7.00013-X
Behrens M, Born S, Redel U, Voigt N, Schuh V, Raguse JD, Meyerhof W (2012) Immunohistochemical detection of TAS2R38 protein in human taste cells. PLoS One 7(7):e40304. doi:10.1371/journal.pone.0040304
Li F (2013) Taste perception: from the tongue to the testis. Mol Hum Reprod 19(6):349–360. doi:10.1093/molehr/gat009
Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK (2013) How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. BioEssays 35(12):1111–1118. doi:10.1002/bies.201300077
Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK (2013) CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495(7440):223–226. doi:10.1038/nature11906
Liman ER, Zhang YV, Montell C (2014) Peripheral coding of taste. Neuron 81(5):984–1000. doi:10.1016/j.neuron.2014.02.022
Roper SD (2013) Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol 24(1):71–79. doi:10.1016/j.semcdb.2012.12.002
Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD (2007) Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27(40):10840–10848. doi:10.1523/JNEUROSCI.1863-07.2007
Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y (2006) Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol 96(6):3088–3095. doi:10.1152/jn.00409.2006
Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S (2010) Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30(25):8376–8382. doi:10.1523/JNEUROSCI.0496-10.2010
Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142(5):687–698. doi:10.1016/j.cell.2010.07.041
Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA (2014) CD36- and GPR120-mediated Ca(2)(+) signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 146(4):995–1005. doi:10.1053/j.gastro.2014.01.006
Sclafani A, Zukerman S, Ackroff K (2013) GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 305(12):R1490–R1497. doi:10.1152/ajpregu.00440.2013
Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P (2011) The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6(8):e24014. doi:10.1371/journal.pone.0024014
Godinot N, Yasumatsu K, Barcos ME, Pineau N, Ledda M, Viton F, Ninomiya Y, le Coutre J, Damak S (2013) Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience 250:20–30. doi:10.1016/j.neuroscience.2013.06.043
Gilbertson TA, Khan NA (2014) Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 53:82–92. doi:10.1016/j.plipres.2013.11.001
Dramane G, Akpona S, Simonin AM, Besnard P, Khan NA (2011) Cell signaling mechanisms of gustatory perception of lipids: can the taste cells be the target of anti-obesity agents? Curr Med Chem 18(22):3417–3422 BSP/CMC/E-Pub/2011/251 [pii]
Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P (2005) CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115(11):3177–3184. doi:10.1172/JCI25299
Khan NA, Besnard P (2009) Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta 1791(3):149–155. doi:10.1016/j.bbalip.2009.01.001
Abdoul-Azize S, Selvakumar S, Sadou H, Besnard P, Khan NA (2014) Ca2 + signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie 96:8–13. doi:10.1016/j.biochi.2013.06.005
Laffitte A, Neiers F, Briand L (2014) Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. doi:10.1097/MCO.0000000000000058
Clark AA, Liggett SB, Munger SD (2012) Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J 26(12):4827–4831. doi:10.1096/fj.12-215087
Depoortere I (2014) Taste receptors of the gut: emerging roles in health and disease. Gut 63(1):179–190. doi:10.1136/gutjnl-2013-305112
Yamamoto K, Ishimaru Y (2013) Oral and extra-oral taste perception. Semin Cell Dev Biol 24(3):240–246. doi:10.1016/j.semcdb.2012.08.005
Gilbertson TA, Damak S, Margolskee RF (2000) The molecular physiology of taste transduction. Curr Opin Neurobiol 10(4):519–527
Mennella JA, Spector AC, Reed DR, Coldwell SE (2013) The bad taste of medicines: overview of basic research on bitter taste. Clin Ther 35(8):1225–1246. doi:10.1016/j.clinthera.2013.06.007
Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA 100(15):8981–8986. doi:10.1073/pnas.1531172100
Sbarbati A, Osculati F (2003) Solitary chemosensory cells in mammals? Cells Tissues Organs 175(1):51–55
Tizzano M, Merigo F, Sbarbati A (2006) Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat 209(3):333–337. doi:10.1111/j.1469-7580.2006.00617.x
Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC (2008) Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol 99(6):2929–2937. doi:10.1152/jn.00066.2008
Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE (2010) Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA 107(7):3210–3215. doi:10.1073/pnas.0911934107
Tizzano M, Cristofoletti M, Sbarbati A, Finger TE (2011) Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 11:3. doi:10.1186/1471-2466-11-3
Braun T, Mack B, Kramer MF (2011) Solitary chemosensory cells in the respiratory and vomeronasal epithelium of the human nose: a pilot study. Rhinology 49(5):507–512. doi:10.4193/Rhin
Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE, Kinnamon SC, Ramakrishnan VR (2013) Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol 3(6):450–457. doi:10.1002/alr.21149
Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, Cohen NA (2014) Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124(3):1393–1405. doi:10.1172/JCI72094
Saunders CJ, Christensen M, Finger TE, Tizzano M (2014) Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci USA 111(16):6075–6080. doi:10.1073/pnas.1402251111
Pearson JP, Passador L, Iglewski BH, Greenberg EP (1995) A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92(5):1490–1494
Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76(1):46–65. doi:10.1128/MMBR.05007-11
Chadwick M, Trewin H, Gawthrop F, Wagstaff C (2013) Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci 14(6):12780–12805. doi:10.3390/ijms140612780
Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias P-T, Ischiropoulos H, Kreindler JL, Reed DR, Cohen NA (2012) T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122(11):4145–4159
Lee RJ, Chen B, Redding KM, Margolskee RF, Cohen NA (2014) Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorum-sensing molecules require taste signaling components. Innate Immun 20(6):606–617. doi:10.1177/1753425913503386
Kim U, Wooding S, Ricci D, Jorde LB, Drayna D (2005) Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat 26(3):199–204. doi:10.1002/humu.20203
Li D, Zhang J (2014) Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol Biol Evol 31(2):303–309. doi:10.1093/molbev/mst219
Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, Duffy VB (2011) Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses 36(3):311–319. doi:10.1093/chemse/bjq132
Lanier SA, Hayes JE, Duffy VB (2005) Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav 83(5):821–831. doi:10.1016/j.physbeh.2004.10.004
Lionakis MS, Netea MG, Holland SM (2014) Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb Perspect Med 4(6):a019638. doi:10.1101/cshperspect.a019638
Greisner WA 3rd, Settipane GA (1996) Hereditary factor for nasal polyps. Allergy Asthma Proc 17(5):283–286
Cohen NA, Widelitz JS, Chiu AG, Palmer JN, Kennedy DW (2006) Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol Head Neck Surg 134(4):601–604. doi:10.1016/j.otohns.2005.11.042
Hamilos DL (2007) Chronic rhinosinusitis patterns of illness. Clin Allergy Immunol 20:1–13
Antunes MB, Gudis DA, Cohen NA (2009) Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am 29(4):631–643. doi:10.1016/j.iac.2009.07.004
Cohen NA (2006) Sinonasal mucociliary clearance in health and disease. Ann Otol Rhinol Laryngol Suppl 196:20–26
Genoway KA, Philpott CM, Javer AR (2011) Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. J Otolaryngol Head Neck Surg 40(3):232–237
Hamilos DL (2013) Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. doi:10.1016/j.jaci.2013.06.049
Kingdom TT, Swain RE Jr (2004) The microbiology and antimicrobial resistance patterns in chronic rhinosinusitis. Am J Otolaryngol 25(5):323–328 S0196070904000341 [pii]
Ooi EH, Wormald PJ, Tan LW (2008) Innate immunity in the paranasal sinuses: a review of nasal host defenses. Am J Rhinol 22(1):13–19. doi:10.2500/ajr.2008.22.3127
Ramanathan M Jr, Lane AP (2007) Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg 136(3):348–356. doi:10.1016/j.otohns.2006.11.011
Lee RJ, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Cohen NA (2013) Vasoactive intestinal peptide regulates sinonasal mucociliary clearance and synergizes with histamine in stimulating sinonasal fluid secretion. FASEB J 27(12):5094–5103. doi:10.1096/fj.13-234476
Zhao KQ, Cowan AT, Lee RJ, Goldstein N, Droguett K, Chen B, Zheng C, Villalon M, Palmer JN, Kreindler JL, Cohen NA (2012) Molecular modulation of airway epithelial ciliary response to sneezing. FASEB J 26(8):3178–3187. doi:10.1096/fj.11-202184
Sleigh MA, Blake JR, Liron N (1988) The propulsion of mucus by cilia. Am Rev Respir Dis 137(3):726–741
Eliezer N, Sade J, Silberberg A, Nevo AC (1970) The role of mucus in transport by cilia. Am Rev Respir Dis 102(1):48–52
Gudis D, Zhao KQ, Cohen NA (2012) Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy 26(1):1–6. doi:10.2500/ajra.2012.26.3716
Gudis DA, Cohen NA (2010) Cilia dysfunction. Otolaryngol Clin North Am 43(3):461–472. doi:10.1016/j.otc.2010.02.007
Knowles MR, Boucher RC (2002) Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109(5):571–577
Parker D, Prince A (2011) Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45(2):189–201. doi:10.1165/rcmb.2011-0011RT
Fang FC (1997) Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99(12):2818–2825. doi:10.1172/JCI119473
Marcinkiewicz J (1997) Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology 37(1):35–41 S0162310996001683 [pii]
Deja M, Busch T, Bachmann S, Riskowski K, Campean V, Wiedmann B, Schwabe M, Hell B, Pfeilschifter J, Falke KJ, Lewandowski K (2003) Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am J Respir Crit Care Med 168(3):281–286. doi:10.1164/rccm.200207-640OC
Degano B, Valmary S, Serrano E, Brousset P, Arnal JF (2011) Expression of nitric oxide synthases in primary ciliary dyskinesia. Hum Pathol 42(12):1855–1861. doi:10.1016/j.humpath.2011.01.027
Haight JS, Djupesland PG, Qjan W, Chatkin JM, Furlott H, Irish J, Witterick I, McClean P, Fenton RS, Hoffstein V, Zamel N (1999) Does nasal nitric oxide come from the sinuses? J Otolaryngol 28(4):197–204
Naraghi M, Deroee AF, Ebrahimkhani M, Kiani S, Dehpour A (2007) Nitric oxide: a new concept in chronic sinusitis pathogenesis. Am J Otolaryngol 28(5):334–337. doi:10.1016/j.amjoto.2006.10.014
Phillips PS, Sacks R, Marcells GN, Cohen NA, Harvey RJ (2011) Nasal nitric oxide and sinonasal disease: a systematic review of published evidence. Otolaryngol Head Neck Surg 144(2):159–169
Ricciardolo FL (2003) Multiple roles of nitric oxide in the airways. Thorax 58(2):175–182
Zhang Y, Endam LM, Filali-Mouhim A, Bosse Y, Castano R, Desrosiers M (2011) Polymorphisms in the nitric oxide synthase 1 gene are associated with severe chronic rhinosinusitis. Am J Rhinol Allergy 25(2):e49–e54. doi:10.2500/ajra.2011.25.3588
Gliklich RE, Metson R (1995) The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 113(1):104–109
Khalid AN, Quraishi SA, Kennedy DW (2004) Long-term quality of life measures after functional endoscopic sinus surgery. Am J Rhinol 18(3):131–136
Hens G, Hellings PW (2006) The nose: gatekeeper and trigger of bronchial disease. Rhinology 44(3):179–187
Bhattacharyya N, Kepnes LJ (2008) Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann Otol Rhinol Laryngol 117(6):448–452
Marcinkiewicz J, Strus M, Pasich E (2013) Antibiotic resistance: a “dark side” of biofilm-associated chronic infections. Pol Arch Med Wewn 123(6):309–313
Rujanavej V, Soudry E, Banaei N, Baron EJ, Hwang PH, Nayak JV (2013) Trends in incidence and susceptibility among methicillin-resistant Staphylococcus aureus isolated from intranasal cultures associated with rhinosinusitis. Am J Rhinol Allergy 27(2):134–137. doi:10.2500/ajra.2013.27.3858
Manes RP, Batra PS (2012) Bacteriology and antibiotic resistance in chronic rhinosinusitis. Facial Plast Surg Clin North Am 20(1):87–91. doi:10.1016/j.fsc.2011.10.010
Kennedy JL, Borish L (2013) Chronic rhinosinusitis and antibiotics: the good, the bad, and the ugly. Am J Rhinol Allergy 27(6):467–472. doi:10.2500/ajra.2013.27.3960
Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A (2004) Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30(6):777–783. doi:10.1165/rcmb.2003-0329OC
Greene CM, McElvaney NG (2005) Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz) 53(5):418–427
Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ (2009) Motile cilia of human airway epithelia are chemosensory. Science 325(5944):1131–1134. doi:10.1126/science.1173869
Singla V, Reiter JF (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313(5787):629–633. doi:10.1126/science.1124534
Satir P, Christensen ST (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129(6):687–693. doi:10.1007/s00418-008-0416-9
Takeda S, Narita K (2012) Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation 83(2):S4–S11. doi:10.1016/j.diff.2011.11.002
Salathe M (2007) Regulation of mammalian ciliary beating. Annu Rev Physiol 69:401–422. doi:10.1146/annurev.physiol.69.040705.141253
Babu D, Roy S (2013) Left-right asymmetry: cilia stir up new surprises in the node. Open Biol 3(5):130052. doi:10.1098/rsob.130052
Lai Y, Chen B, Shi J, Palmer JN, Kennedy DW, Cohen NA (2011) Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol 128(6):e11207–e11215. doi:10.1016/j.jaci.2011.09.001
Ramanathan M Jr, Lane AP (2007) A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol 21(3):373–377
Antunes MB, Woodworth BA, Bhargave G, Xiong G, Aguilar JL, Ratner AJ, Kreindler JL, Rubenstein RC, Cohen NA (2007) Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques 43(2):195–196 198, 200 passim 000112531 [pii]
Dimova S, Brewster ME, Noppe M, Jorissen M, Augustijns P (2005) The use of human nasal in vitro cell systems during drug discovery and development. Toxicol In Vitro 19(1):107–122. doi:10.1016/j.tiv.2004.07.003
Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA (2007) Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol 21(5):533–537. doi:10.2500/ajr.2007.21.3068
Perez CA, Margolskee RF, Kinnamon SC, Ogura T (2003) Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium 33(5–6):541–549
Zhang Z, Zhao Z, Margolskee R, Liman E (2007) The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 27(21):5777–5786. doi:10.1523/JNEUROSCI.4973-06.2007
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5(11):1169–1176. doi:10.1038/nn952
Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S (1991) Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 181(2):852–857
Maniscalco M, Sofia M, Pelaia G (2007) Nitric oxide in upper airways inflammatory diseases. Inflamm Res 56(2):58–69. doi:10.1007/s00011-006-6111-1
Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179(18):5756–5767
Li Z, Nair SK (2012) Quorum sensing: How bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. doi:10.1002/pro.2132
Whitney G, Harder DB (1994) Genetics of bitter perception in mice. Physiol Behav 56(6):1141–1147
Wu SV, Chen MC, Rozengurt E (2005) Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics 22(2):139–149. doi:10.1152/physiolgenomics.00030.2005
Whitney G, Harder DB (1986) Phenylthiocarbamide (PTC) preference among laboratory mice: understanding of a previously “unreplicated” report. Behav Genet 16(6):605–610
St John SJ, Pour L, Boughter JD Jr (2005) Phenylthiocarbamide produces conditioned taste aversions in mice. Chem Senses 30(5):377–382 bji032 [pii] 0.1093/chemse/bji032
Nelson TM, Munger SD, Boughter JD Jr (2003) Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses 28(8):695–704
Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99(4):2392–2397. doi:10.1073/pnas.042617699
Chen MC, Wu SV, Reeve JR Jr, Rozengurt E (2006) Bitter stimuli induce Ca2 + signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2 + channels. Am J Physiol Cell Physiol 291(4):C726–C739. doi:10.1152/ajpcell.00003.2006
Jeon TI, Seo YK, Osborne TF (2011) Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J 438(1):33–37. doi:10.1042/BJ20110009
Ruiz-Avila L, Wong GT, Damak S, Margolskee RF (2001) Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha -gustducin. Proc Natl Acad Sci USA 98(15):8868–8873. doi:10.1073/pnas.151235798
Hoon MA, Northup JK, Margolskee RF, Ryba NJ (1995) Functional expression of the taste specific G-protein, alpha-gustducin. Biochem J 309(Pt 2):629–636
McLaughlin SK, McKinnon PJ, Margolskee RF (1992) Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357(6379):563–569. doi:10.1038/357563a0
Caicedo A, Pereira E, Margolskee RF, Roper SD (2003) Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci 23(30):9947–9952
He W, Danilova V, Zou S, Hellekant G, Max M, Margolskee RF, Damak S (2002) Partial rescue of taste responses of alpha-gustducin null mice by transgenic expression of alpha-transducin. Chem Senses 27(8):719–727
Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC (2005) Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses 30(4):299–316. doi:10.1093/chemse/bji025
Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W (2005) The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol 15(4):322–327. doi:10.1016/j.cub.2005.01.047
Bachmanov AA, Bosak NP, Lin C, Matsumoto I, Ohmoto M, Reed DR, Nelson TM (2014) Genetics of taste receptors. Curr Pharm Des 20(16):2669–2683 CPD-E-PUB-54566 [pii]
Guo SW, Reed DR (2001) The genetics of phenylthiocarbamide perception. Ann Hum Biol 28(2):111–142
Mennella JA, Pepino MY, Reed DR (2005) Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115(2):e216–e222. doi:10.1542/peds.2004-1582
Reed DR, Knaapila A (2010) Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci 94:213–240. doi:10.1016/B978-0-12-375003-7.00008-X
Tan J, Abrol R, Trzaskowski B, Goddard WA 3rd (2012) 3D Structure Prediction of TAS2R38 Bitter Receptors Bound to Agonists Phenylthiocarbamide (PTC) and 6-n-Propylthiouracil (PROP). J Chem Inf Model. doi:10.1021/ci300133a
Biarnes X, Marchiori A, Giorgetti A, Lanzara C, Gasparini P, Carloni P, Born S, Brockhoff A, Behrens M, Meyerhof W (2010) Insights into the binding of Phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PLoS One 5(8):e12394. doi:10.1371/journal.pone.0012394
Floriano WB, Hall S, Vaidehi N, Kim U, Drayna D, Goddard WA 3rd (2006) Modeling the human PTC bitter-taste receptor interactions with bitter tastants. J Mol Model 12(6):931–941. doi:10.1007/s00894-006-0102-6
Lipchock SV, Mennella JA, Spielman AI, Reed DR (2013) Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am J Clin Nutr 98(4):1136–1143. doi:10.3945/ajcn.113.066688
Adappa ND, Howland TJ, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, Reed DR, Lee RJ, Cohen NA (2013) Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol 3(3):184–187
Adappa ND, Zhang Z, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, Reed DR, Scott T, Zhao NW, Owens D, Lee RJ, Cohen NA (2013) The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. doi:10.1002/alr.21253
Mfuna Endam L, Filali-Mouhim A, Boisvert P, Boulet LP, Bosse Y, Desrosiers M (2014) Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol 4(3):200–206. doi:10.1002/alr.21275
Sbarbati A, Merigo F, Osculati F (2010) Eukaryotic vs. prokaryotic chemosensory systems. Biomed Pharmacother 64(4):233–239. doi:10.1016/j.biopha.2009.06.015
Gulbransen B, Silver W, Finger TE (2008) Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol 508(1):62–71. doi:10.1002/cne.21657
Osculati F, Bentivoglio M, Castellucci M, Cinti S, Zancanaro C, Sbarbati A (2007) The solitary chemosensory cells and the diffuse chemosensory system of the airway. Eur J Histochem 51(Suppl 1):65–72
Kotrschal K (2000) Taste(s) and olfaction(s) in fish: a review of specialized sub-systems and central integration. Pflugers Arch 439(3 Suppl):R178–R180
Tizzano M, Finger TE (2013) Chemosensors in the nose: guardians of the airways. Physiology (Bethesda) 28(1):51–60. doi:10.1152/physiol.00035.2012
Whitear M (1992) Solitary chemoreceptor cells. In: Hara TJ (ed) Chemoreception in Fishes. Chapman and Hall, London, pp 103–125
Hansen A (2007) Olfactory and solitary chemosensory cells: two different chemosensory systems in the nasal cavity of the American alligator, Alligator mississippiensis. BMC Neurosci 8:64. doi:10.1186/1471-2202-8-64
Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D (2008) TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol 99(3):1451–1460. doi:10.1152/jn.01195.2007
Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schutz B, Kotlikoff M, Ibanez-Tallon I, Kummer W (2011) Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA 108(23):9478–9483. doi:10.1073/pnas.1019418108
Baraniuk JN, Kaliner MA (1990) Neuropeptides and nasal secretion. J Allergy Clin Immunol 86(4 Pt 2):620–627
Dinh QT, Groneberg DA, Mingomataj E, Peiser C, Heppt W, Dinh S, Arck PC, Klapp BF, Fischer A (2003) Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides 37(4):245–250 S0143417903000659 [pii]
Fang SY, Shen CL (1997) Neuropeptidergic innervation of human nasal mucosa in various pathological conditions. Proc Natl Sci Counc Repub China B 21(1):8–12
Mendonca JC, Dolci JE (2005) Neuropeptide immunofluorescence in human nasal mucosa: assessment of the technique for vasoactive intestinal peptide (VIP). Braz J Otorhinolaryngol 71(2):123–131 S0034-72992005000200002 [pii]/S0034-72992005000200002
Mosimann BL, White MV, Hohman RJ, Goldrich MS, Kaulbach HC, Kaliner MA (1993) Substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol 92(1 Pt 1):95–104
Ianowski JP, Choi JY, Wine JJ, Hanrahan JW (2007) Mucus secretion by single tracheal submucosal glands from normal and CFTR knock-out mice. J Physiol 580:301–314
Ianowski JP, Choi JY, Wine JJ, Hanrahan JW (2008) Substance P stimulates CFTR-dependent fluid secretion by mouse tracheal submucosal glands. Pflugers Arch 457:529–537
Lee RJ, Foskett JK (2010) cAMP-activated Ca2 + signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest 120(9):3137–3148
Lee RJ, Foskett JK (2012) Why mouse airway submucosal gland serous cells do not secrete fluid in response to cAMP stimulation. J Biol Chem 287(45):38316–38326. doi:10.1074/jbc.M112.412817
Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35(2):157–170. doi:10.1093/chemse/bjp092
Pereira CS, Thompson JA, Xavier KB (2013) AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37(2):156–181. doi:10.1111/j.1574-6976.2012.00345.x
Frederix M, Downie AJ (2011) Quorum sensing: regulating the regulators. Adv Microb Physiol 58:23–80. doi:10.1016/B978-0-12-381043-4.00002-7
Abraham WR (2006) Controlling biofilms of gram-positive pathogenic bacteria. Curr Med Chem 13(13):1509–1524
Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M (2005) Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem 280(15):15238–15246. doi:10.1074/jbc.M414287200
Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, Max M, Margolskee RF (2005) Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J Biol Chem 280(40):34296–34305. doi:10.1074/jbc.M505255200
Imada T, Misaka T, Fujiwara S, Okada S, Fukuda Y, Abe K (2010) Amiloride reduces the sweet taste intensity by inhibiting the human sweet taste receptor. Biochem Biophys Res Commun 397(2):220–225. doi:10.1016/j.bbrc.2010.05.088
Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF (2003) Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301(5634):850–853. doi:10.1126/science.1087155
Lemon CH, Margolskee RF (2009) Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101(5):2459–2471. doi:10.1152/jn.90892.2008
Chiu AG, Antunes MB, Feldman M, Cohen NA (2007) An animal model for the study of topical medications in sinusitis. Am J Rhinol 21(1):5–9
Ha KR, Psaltis AJ, Tan L, Wormald PJ (2007) A sheep model for the study of biofilms in rhinosinusitis. Am J Rhinol 21(3):339–345
Wang JC, Hathorn I, Habib AR, Chang E, Javer AR (2013) Evaluation of domestic and Yucatan swine nasal sinus anatomy as models for future sinonasal research of medications delivered by standard instruments used in functional endoscopic sinus surgery. Int Forum Allergy Rhinol 3(2):150–156. doi:10.1002/alr.21081
Trout L, Corboz MR, Ballard ST (2001) Mechanism of substance P-induced liquid secretion across bronchial epithelium. Am J Physiol Lung Cell Mol Physiol 281(3):L639–L645
Choi JY, Khansaheb M, Joo NS, Krouse ME, Robbins RC, Weill D, Wine JJ (2009) Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process. J Clin Invest 119(5):1189–1200
Schiffman SS, Booth BJ, Sattely-Miller EA, Graham BG, Gibes KM (1999) Selective inhibition of sweetness by the sodium salt of ± 2-(4-methoxyphenoxy)propanoic acid. Chem Senses 24(4):439–447
Kyriazis GA, Soundarapandian MM, Tyrberg B (2012) Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 109(8):E524–E532. doi:10.1073/pnas.1115183109
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104(38):15069–15074. doi:10.1073/pnas.0706890104
Garnett JP, Baker EH, Baines DL (2012) Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. Eur Respir J 40(5):1269–1276. doi:10.1183/09031936.00052612
Kalsi KK, Baker EH, Fraser O, Chung YL, Mace OJ, Tarelli E, Philips BJ, Baines DL (2009) Glucose homeostasis across human airway epithelial cell monolayers: role of diffusion, transport and metabolism. Pflugers Arch 457(5):1061–1070. doi:10.1007/s00424-008-0576-4
Pezzulo AA, Gutierrez J, Duschner KS, McConnell KS, Taft PJ, Ernst SE, Yahr TL, Rahmouni K, Klesney-Tait J, Stoltz DA, Zabner J (2011) Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One 6(1):e16166. doi:10.1371/journal.pone.0016166
Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, Hodson ME, Philips BJ, Baines DL (1985) Wood DM (2007) Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol 102(5):1969–1975. doi:10.1152/japplphysiol.01425.2006
Rogers GA, Den Beste K, Parkos CA, Nusrat A, Delgaudio JM, Wise SK (2011) Epithelial tight junction alterations in nasal polyposis. Int Forum Allergy Rhinol 1(1):50–54. doi:10.1002/alr.20014
Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA (2012) Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol 130(5):1087–1096, e1010. doi:10.1016/j.jaci.2012.05.052
Koziel H, Koziel MJ (1995) Pulmonary complications of diabetes mellitus pneumonia. Infect Dis Clin North Am 9(1):65–96
Zhang Z, Adappa ND, Lautenbach E, Chiu AG, Doghramji L, Howland TJ, Cohen NA, Palmer JN (2014) The effect of diabetes mellitus on chronic rhinosinusitis and sinus surgery outcome. Int Forum Allergy Rhinol. doi:10.1002/alr.21269
Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D (2009) Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol 19(15):1288–1293. doi:10.1016/j.cub.2009.06.015
Krasteva G, Canning BJ, Papadakis T, Kummer W (2012) Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci 91(21–22):992–996. doi:10.1016/j.lfs.2012.06.014
Saunders CJ, Reynolds SD, Finger TE (2013) Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir Cell Mol Biol 49(2):190–196. doi:10.1165/rcmb.2012-0485OC
Sbarbati A, Osculati F (2005) A new fate for old cells: brush cells and related elements. J Anat 206(4):349–358. doi:10.1111/j.1469-7580.2005.00403.x
An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA Jr, Liggett SB (2012) TAS2R activation promotes airway smooth muscle relaxation despite beta(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol 303(4):L304–L311. doi:10.1152/ajplung.00126.2012
Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16(11):1299–1304. doi:10.1038/nm.2237
Robinett KS, Deshpande DA, Malone MM, Liggett SB (2011) Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol 45(5):1069–1074. doi:10.1165/rcmb.2011-0061OC
Robinett KS, Koziol-White CJ, Akoluk A, An SS, Panettieri RA Jr, Liggett SB (2014) Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol 50(4):678–683. doi:10.1165/rcmb.2013-0439RC
Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J (2011) Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 469(7330):419–423. doi:10.1038/nature09674
Wilson SS, Wiens ME, Smith JG (2013) Antiviral mechanisms of human defensins. J Mol Biol 425(24):4965–4980. doi:10.1016/j.jmb.2013.09.038
Lee RJ, Cohen NA (2013) The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am J Rhinol Allergy 27(4):283–286. doi:10.2500/ajra.2013.27.3911
Cohen SP, Buckley BK, Kosloff M, Garland AL, Bosch DE, Cheng G Jr, Radhakrishna H, Brown MD, Willard FS, Arshavsky VY, Tarran R, Siderovski DP, Kimple AJ (2012) Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J Biol Chem 287(50):41706–41719. doi:10.1074/jbc.M112.423806
Pulkkinen V, Manson ML, Safholm J, Adner M, Dahlen SE (2012) The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol 303(11):L956–L966. doi:10.1152/ajplung.00205.2012
Grassin-Delyle S, Abrial C, Fayad-Kobeissi S, Brollo M, Faisy C, Alvarez JC, Naline E, Devillier P (2013) The expression and relaxant effect of bitter taste receptors in human bronchi. Respir Res 14:134. doi:10.1186/1465-9921-14-134
Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, Schutz B, Langheinrich AC, Chubanov V, Gudermann T, Ibanez-Tallon I, Kummer W (2012) Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol 137(4):483–497. doi:10.1007/s00418-012-0911-x
Elliott RA, Kapoor S, Tincello DG (2011) Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol 186(6):2455–2462. doi:10.1016/j.juro.2011.07.083
Dehkordi O, Rose JE, Fatemi M, Allard JS, Balan KV, Young JK, Fatima S, Millis RM, Jayam-Trouth A (2012) Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res 1475:1–10. doi:10.1016/j.brainres.2012.07.038
Singh N, Vrontakis M, Parkinson F, Chelikani P (2011) Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun 406(1):146–151. doi:10.1016/j.bbrc.2011.02.016
Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE (2009) Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3:12. doi:10.3389/neuro.07.012.2009
Shin YJ, Park JH, Choi JS, Chun MH, Moon YW, Lee MY (2010) Enhanced expression of the sweet taste receptors and alpha-gustducin in reactive astrocytes of the rat hippocampus following ischemic injury. Neurochem Res 35(10):1628–1634. doi:10.1007/s11064-010-0223-2
Singh N, Chakraborty R, Bhullar RP, Chelikani P (2014) Differential expression of bitter taste receptors in non-cancerous breast epithelial and breast cancer cells. Biochem Biophys Res Commun 446(2):499–503. doi:10.1016/j.bbrc.2014.02.140
Foster SR, Porrello ER, Purdue B, Chan HW, Voigt A, Frenzel S, Hannan RD, Moritz KM, Simmons DG, Molenaar P, Roura E, Boehm U, Meyerhof W, Thomas WG (2013) Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One 8(5):e64579. doi:10.1371/journal.pone.0064579
Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L (2009) Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 296(3):R528–R536. doi:10.1152/ajpregu.90423.2008
Dotson CD, Zhang L, Xu H, Shin YK, Vigues S, Ott SH, Elson AE, Choi HJ, Shaw H, Egan JM, Mitchell BD, Li X, Steinle NI, Munger SD (2008) Bitter taste receptors influence glucose homeostasis. PLoS One 3(12):e3974. doi:10.1371/journal.pone.0003974
Bezencon C, le Coutre J, Damak S (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32(1):41–49. doi:10.1093/chemse/bjl034
Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I (2011) Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108(5):2094–2099. doi:10.1073/pnas.1011508108
Kokrashvili Z, Mosinger B, Margolskee RF (2009) Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90(3):822S–825S. doi:10.3945/ajcn.2009.27462T
Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33(Pt 1):302–305. doi:10.1042/BST0330302
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na + -glucose cotransporter 1. Proc Natl Acad Sci USA 104(38):15075–15080. doi:10.1073/pnas.0706678104
Sclafani A (2007) Sweet taste signaling in the gut. Proc Natl Acad Sci USA 104(38):14887–14888. doi:10.1073/pnas.0707410104
Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP (2010) Expression of Na +/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr 104(5):637–646. doi:10.1017/S0007114510000917
Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C (2011) The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 30(4):524–532. doi:10.1016/j.clnu.2011.01.007
Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C (2011) The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab 301(2):E317–E325. doi:10.1152/ajpendo.00077.2011
Geraedts MC, Takahashi T, Vigues S, Markwardt ML, Nkobena A, Cockerham RE, Hajnal A, Dotson CD, Rizzo MA, Munger SD (2012) Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab 303(4):E464–E474. doi:10.1152/ajpendo.00163.2012
Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, Bravo D (2014) Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr 1:8. doi:10.1017/S0007114513002286
Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C (2014) Gut sweet taste receptors and their role in metabolism. Front Horm Res 42:123–133. doi:10.1159/000358321
Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F (1998) Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell Signal 10(10):727–733
Xu J, Cao J, Iguchi N, Riethmacher D, Huang L (2013) Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod 19(1):17–28. doi:10.1093/molehr/gas040
Li F, Zhou M (2012) Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol Hum Reprod 18(6):289–297. doi:10.1093/molehr/gas005
Meyer D, Voigt A, Widmayer P, Borth H, Huebner S, Breit A, Marschall S, de Angelis MH, Boehm U, Meyerhof W, Gudermann T, Boekhoff I (2012) Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca(2)(+) and cAMP concentrations in spermatozoa. PLoS One 7(2):e32354. doi:10.1371/journal.pone.0032354
Mosinger B, Redding KM, Parker MR, Yevshayeva V, Yee KK, Dyomina K, Li Y, Margolskee RF (2013) Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc Natl Acad Sci USA 110(30):12319–12324. doi:10.1073/pnas.1302827110
Voigt A, Hubner S, Lossow K, Hermans-Borgmeyer I, Boehm U, Meyerhof W (2012) Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem Senses 37(9):897–911. doi:10.1093/chemse/bjs082
Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2(12):1055–1062. doi:10.1038/15981
Giovannucci DR, Groblewski GE, Sneyd J, Yule DI (2000) Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2 + release and shapes oscillatory Ca2 + signals. J Biol Chem 275(43):33704–33711. doi:10.1074/jbc.M004278200
Yule DI, Straub SV, Bruce JI (2003) Modulation of Ca2 + oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans 31(Pt 5):954–957
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC (2001) Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2:6
Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K (2007) Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem 282(51):37225–37231. doi:10.1074/jbc.M705641200
Miyoshi MA, Abe K, Emori Y (2001) IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses 26(3):259–265
Gao N, Lu M, Echeverri F, Laita B, Kalabat D, Williams ME, Hevezi P, Zlotnik A, Moyer BD (2009) Voltage-gated sodium channels in taste bud cells. BMC Neurosci 10:20. doi:10.1186/1471-2202-10-20
Waterer GW (2012) Airway defense mechanisms. Clin Chest Med 33(2):199–209. doi:10.1016/j.ccm.2012.03.003
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837. doi:10.1093/eurheartj/ehr304 837a-837d
Shaul PW (2002) Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64:749–774. doi:10.1146/annurev.physiol.64.081501.155952
Stout SL, Wyatt TA, Adams JJ, Sisson JH (2007) Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem 55(5):433–442. doi:10.1369/jhc.6A7089.2007
Acknowledgments
Some of the research described in this review was supported by a grant from the Flight Attendants Medical Research Institute (082478) and a philanthropic contribution from the RLG Foundation Inc., both to N.A.C.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, R.J., Cohen, N.A. Taste receptors in innate immunity. Cell. Mol. Life Sci. 72, 217–236 (2015). https://doi.org/10.1007/s00018-014-1736-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1736-7