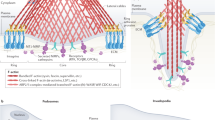
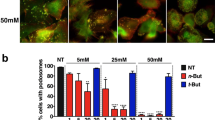
Abstract
Podosomes are adhesion and invasion structures that are particularly prominent in cells of the monocytic lineage such as macrophages, dendritic cells, and osteoclasts. They are multifunctional organelles that combine several key abilities required for cell migration and invasion. The podosome repertoire includes well-established functions such as cell-substrate adhesion, and extracellular matrix degradation, recently discovered abilities such as rigidity and topology sensing as well as antigen sampling, and also more speculative functions such as cell protrusion stabilization and transmigration. Collectively, podosomes not only enable dynamic interactions of cells with their surroundings, they also gather information about the pericellular environment, and are actively involved in its reshaping. This review presents an overview of the current knowledge on podosome composition, architecture, and regulation. We focus in particular on the growing list of podosome functions and discuss the specific properties of podosomes in macrophages, dendritic cells, and osteoclasts. Moreover, this article highlights podosome-related intracellular transport processes, the formation of podosomes in 3D environments as well as potentially podosome-associated diseases involving monocytic cells.





Similar content being viewed by others
Abbreviations
- ADAM:
-
A disintegrin and metalloproteinase
- Arp:
-
Actin-related protein
- CD:
-
Cluster of differentiation
- ECM:
-
Extracellular matrix
- FMNL:
-
Formin-like
- HDAC:
-
Histone deacetylase
- KIF:
-
Kinesin-like
- LAD:
-
Leukocyte adhesion deficiency
- MMP:
-
Matrix metalloproteinase
- MT1-MMP:
-
Membrane-type 1-matrix metalloproteinase
- PAPA:
-
Pyogenic sterile arthritis, pyoderma gangrenosum, and acne (syndrome)
- PSTPIP:
-
Proline-serine-threonine phosphatase interacting protein
- PMA:
-
Phorbol 12-myristate 13-acetate
- SZ:
-
Sealing zone
- TAM:
-
Tumor-associated macrophage
- WAS:
-
Wiskott-Aldrich syndrome
- WASP:
-
Wiskott-Aldrich syndrome protein
References
Ridley AJ (2011) Life at the leading edge. Cell 145(7):1012–1022
Zaidel-Bar R, Milo R, Kam Z, Geiger B (2007) A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci 120(Pt 1):137–148
Linder S, Wiesner C, Himmel M (2011) Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol 27:185–211
Murphy DA, Courtneidge SA (2011) The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 12(7):413–426
Linder S, Nelson D, Weiss M, Aepfelbacher M (1999) Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci USA 96:9648–9653
Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE (2001) Configuration of human dendritic cell cytoskeleton by Rho GTPases, theWAS protein, and differentiation. Blood 98:1142–1149
Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F (2003) Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell 14:407–416
Moreau V, Tatin F, Varon C, Genot E (2003) Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol 23(19):6809–6822
Osiak AE, Zenner G, Linder S (2005) Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp Cell Res 307:342–353
Burgstaller G, Gimona M (2004) Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci 117(2):223–231
Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J (2004) Imaging sites of N-WASP activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol 14(8):697–703
Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen WT (1994) Apotential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res 54(21):5702–5710
Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC (1985) Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res 159(1):141–157
Linder S (2007) The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 17(3):107–117
Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J (2010) IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep 23(6):1553–1559
Linder S, Hufner K, Wintergerst U, Aepfelbacher M (2000) Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J Cell Sci 113:4165–4176
van den Dries K, van Helden SF, Jt Riet, Diez-Ahedo R, Manzo C, Oud MM, van Leeuwen FN, Brock R, Garcia-Parajo MF, Cambi A, Figdor CG (2012) Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cell Mol Life Sci 69(11):1889–1901
Gawden-Bone C, Zhou Z, King E, Prescott A, Watts C, Lucocq J (2010) Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J Cell Sci 123(Pt 9):1427–1437
Destaing O, Block MR, Planus E, Albiges-Rizo C (2011) Invadosome regulation by adhesion signaling. Curr Opin Cell Biol 23(5):597–606
Hoshino D, Branch KM, Weaver AM (2013) Signaling inputs to invadopodia and podosomes. J Cell Sci 126(Pt 14):2979–2989
Spuul P, Ciufici P, Veillat V, Leclercq A, Daubon T, Kramer I, Génot E (2014) Importance of RhoGTPases in formation, characteristics, and functions of invadosomes. Small GTPases 5:e28713
Revach OY, Geiger B (2013) The interplay between the proteolytic, invasive, and adhesive domains of invadopodia and their roles in cancer invasion. Cell Adh Migr 8(3)
Marchisio PC (2012) Fortuitous birth, convivial baptism and early youth of podosomes. Eur J Cell Biol 91(11–12):820–823
Linder S (2009) Invadosomes at a glance. J Cell Sci 122:3009–3013
Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charriere GM (2010) Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci USA 107(49):21016–21021
Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, De Camilli P (2000) A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol 150(2):377–389
Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska KA (2000) Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol 148(4):665–678
Zambonin-Zallone A, Teti A, Grano M, Rubinacci A, Abbadini M, Gaboli M, Marchisio PC (1989) Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: a β3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp Cell Res 182:645–652
Pfaff M, Jurdic P (2001) Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J Cell Sci 114(Pt 15):2775–2786
Linder S, Aepfelbacher M (2003) Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 13(7):376–385
Cox S, Rosten E, Monypenny J, Jovanovic-Talisman T, Burnette DT, Lippincott-Schwartz J, Jones GE, Heintzmann R (2011) Bayesian localisation microscopy reveals nanoscale podosome dynamics. Nat Methods 9(2):195–200
van den Dries K, Schwartz SL, Byars J, Meddens MB, Bolomini-Vittori M, Lidke DS, Figdor CG, Lidke KA, Cambi A (2013) Dual-color superresolution microscopy reveals nanoscale organization of mechanosensory podosomes. Mol Biol Cell 24(13):2112–2123
Walde M, Monypenny J, Heintzmann R, Jones GE, Cox S (2014) Vinculin binding angle in podosomes revealed by high resolution microscopy. PLoS One 9(2):e88251
Linder S, Higgs H, Hufner K, Schwarz K, Pannicke U, Aepfelbacher M (2000) The polarization defect of Wiskott-Aldrich syndrome macrophages is linked to dislocalization of the Arp2/3 complex. J Immunol 165:221–225
Kaverina I, Stradal TE, Gimona M (2003) Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci 116(24):4915–4924
Akisaka T, Yoshida H, Suzuki R, Takama K (2008) Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell Tissue Res 331(3):625–641
Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L (2007) The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One 2(1):e179
Bhuwania R, Cornfine S, Fang Z, Krüger M, Luna EJ, Linder S (2012) Supervillin couples myosin-dependent contractility to podosomes and enables their turnover. J Cell Sci 125(Pt 9):2300–2314
Mersich AT, Miller MR, Chkourko H, Blystone SD (2010) The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton 67(9):573–585
Kopp P, Lammers R, Aepfelbacher M, Woehlke G, Rudel T, Machuy N, Steffen W, Linder S (2006) The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol Biol Cell 17(6):2811–2823
van Helden SF, Oud MM, Joosten B, Peterse N, Figdor CG, van Leeuwen FN (2008) PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J Cell Sci 121(7):1096–1106
Linder S, Kopp P (2005) Podosomes at a glance. J Cell Sci 118(P10):2079–2082
Teti A, Grano M, Carano A, Colucci S, Zambonin Zallone A (1989) Immunolocalization of beta 3 subunit of integrins in osteoclast membrane. Boll Soc Ital Biol Sper 65(11):1031–1037
Chabadel A, Banon-Rodriguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Génot E, Jurdic P, Anton IM, Saltel F (2007) CD44 and β3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell 18(12):4899–4910
Cervero P, Panzer L, Linder S (2013) Podosome reformation in macrophages: assays and analysis. Methods Mol Biol 1046:97–121
Meddens MB, Rieger B, Figdor CG, Cambi A, van den Dries K (2013) Automated podosome identification and characterization in fluorescence microscopy images. Microsc Microanal 19(1):180–189
Cougoule C, Van Goethem E, Le Cabec V, Lafouresse F, Dupré L, Mehraj V, Mège JL, Lastrucci C, Maridonneau-Parini I (2012) Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur J Cell Biol 91(11–12):938–949
Cougoule C, Le Cabec V, Poincloux R, Al Saati T, Mège JL, Tabouret G, Lowell CA, Laviolette-Malirat N, Maridonneau-Parini I (2010) Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis. Blood 115(7):1444–1452
Burger KL, Davis AL, Isom S, Mishra N, Seals DF (2011) The podosome marker protein Tks5 regulates macrophage invasive behavior. Cytoskeleton (Hoboken) 68(12):694–711
De Clercq S, Boucherie C, Vandekerckhove J, Gettemans J, Guillabert A (2013) L-plastin nanobodies perturb matrix degradation, podosome formation, stabilityand lifetime in THP-1 macrophages. PLoS One 8(11):e78108
Calle Y, Carragher NO, Thrasher AJ, Jones GE (2006) Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci 119(11):2375–2385
West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C (2004) Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 305:1153–1157
Wiesner C, Faix J, Himmel M, Bentzien F, Linder S (2010) KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding and extracellular matrix degradation in primary macrophages. Blood 116:1559–1569
Cornfine S, Himmel M, Kopp P, el Azzouzi K, Wiesner C, Krüger M, Rudel T, Linder S (2011) The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes. Mol Biol Cell 22(2):202–215
Yamaguchi H, Pixley F, Condeelis J (2006) Invadopodia and podosomes in tumor invasion. Eur J Cell Biol 85(3–4):213–218
Baranov MV, Ter Beest M, Reinieren-Beeren I, Cambi A, Figdor CG, van den Bogaart CG (2014) Podosomes of dendritic cells facilitate antigen sampling. J Cell Sci 127(Pt 5):1052–1064
Evans J, Correia I, Krasavina O, Watson N, Matsudaira P (2003) Macrophage podosomes assemble at the leading lamella by growth and fragmentation. J Cell Biol 161:697–705
Cervero P, Himmel M, Krüger M, Linder S (2012) Proteomic analysis of podosome fractions from macrophages reveals similarities to spreading initiation centres. Eur J Cell Biol 91(11–12):908–922
Attanasio F, Caldieri G, Giacchetti G, vanHorssen R, Wieringa B, Buccione R (2011) Novel invadopodia components revealed by differential proteomic analysis. Eur J Cell Biol 90(2–3):115–127
Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, LeCabec V (2010) Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol 184(2):1049–1061
Starnes TW, Bennin DA, Bing X, Eickhoff JC, Grahf DC, Bellak JM, Seroogy CM, Ferguson PJ, Huttenlocher A (2014) The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood 123(17):2703–2714
Burns S, Hardy SJ, Buddle J, Yong KL, Jones GE, Thrasher AJ (2004) Maturation of DC is associated with changes in motile characteristics and adherence. Cell Motil Cytoskeleton 57(2):118–132
Jurdic P, Saltel F, Chabadel A, Destaing O (2006) Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol 85:195–202
Väänänen HK, Zhao H, Mulari M, Halleen JM (2000) The cell biology of osteoclast function. J Cell Sci 113(3):377–381
Saltel F, Chabadel A, Bonnelye E, Jurdic P (2008) Actin cytoskeletal organisation in osteoclasts: a model to decipher transmigration and matrix degradation. Eur J Cell Biol 87:459–468
Geblinger D, Zink C, Spencer ND, Addadi L, Geiger B (2012) Effects of surface microtopography on the assembly of the osteoclast resorption apparatus. J R Soc Interface 9(72):1599–1608
Lakkakorpi PT, Väänänen HK (1991) Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J Bone Miner Res 6(8):817–826
Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, Shibasaki Y, Morii N, Narumiya S, Takahashi N et al (1995) The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci 108(Pt 6):2285–2292
Sato T, del Ovejero Carmen M, Hou P, Heegaard AM, Kumegawa M, Foged MT, Delaissé JM (1997) Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J Cell Sci 110(Pt. 5):589–596
Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M (2003) From lysosomes to the plasma membrane: localization of vacuolar-type H±ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278(24):22023–22030
Bonnelye E, Saltel F, Chabadel A, Zirngibl RA, Aubin JE, Jurdic P (2010) Involvement of the orphan nuclear estrogen receptor-related receptor α in osteoclast adhesion and transmigration. J Mol Endocrinol 45(6):365–377
Zambonin-Zallone A, Teti A, Carano A, Marchisio PC (1988) The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res 3(5):517–523
Destaing O, Saltel F, Gillquin B, Chabadel A, Khochbin S, Ory S, Jurdic P (2005) A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci 118:2901–2911
Biosse Duplan M, Zalli D, Stephens S, Zenger S, Neff L, Oelkers JM, Lai FP, Horne W, Rottner K, Baron R (2014) Microtubule dynamic instability controls podosome patterning in osteoclasts through EB1, cortactin, and Src. Mol Cell Biol 34(1):16–29
Luxenburg C, Winograd-Katz S, Addadi L, Geiger B (2012) Involvement of actin polymerization in podosome dynamics. J Cell Sci 125:1666–1672
Saltel F, Chabadel A, Zhao Y, Lafage-Proust MH, Clézardin P, Jurdic P, Bonnelye E (2006) Transmigration: a new property of mature multinucleated osteoclasts. J Bone Miner Res 21(12):1913–1923
Ory S, Brazier H, Pawlak G, Blangy A (2008) Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol 87:469–477
Touaitahuata H, Blangy A, Vives V (2014) Modulation of osteoclast differentiation and bone resorption by Rho GTPases. Small GTPases 5:e28119
Brazier H, Pawlak G, Vives V, Blangy A (2009) The Rho GTPase Wrch1 regulates osteoclast precursor adhesion and migration. Int J Biochem Cell Biol 41(6):1391–1401
Georgess D, Machuca-Gayet I, Blangy A, Jurdic P (2014) Podosome organization drives osteoclast-mediated bone resorption. Cell Adh Migr 8(3)
Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J (2007) Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/−mice. J Cell Biol 178(6):1053–1064
Chellaiah MA (2006) Regulation of podosomes by integrinαvβ3 and RhoGTPase-facilitated phosphoinositide signaling. Eur J Cell Biol 85(3–4):311–317
Gimona M, Buccione R, Courtneidge SA, Linder S (2008) Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol 20(2):235–241
Legate KR, Fässler R (2009) Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci 122(Pt 2):187–198
Collin O, Tracqui P, Stephanou A, Usson Y, Clément-Lacroix J, Planus E (2006) Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J Cell Sci 119(9):1914–1925
Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N (2008) Self-organized podosomes are dynamic mechanosensors. Curr Biol 18(17):1288–1294
van den Dries K, Bolomini-Vittori M, Cambi A (2014) Spatiotemporal organization and mechanosensory function of podosomes. Cell Adh Migr 8(3)
Schiller HB, Fässler R (2013) Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep 14(6):509–519
Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. Curr Protoc Immunol. Chapter 14:Unit 14.1
Margadant F, Chew LL, Hu X, Yu H, Bate N, Zhang X, Sheetz M (2011) Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol 12:e1001223
Schmidt S, Nakchbandi I, Ruppert R, Kawelke N, Hess MW, Pfaller K, Jurdic P, Fässler R, Moser M (2011) Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J Cell Biol 192(5):883–897
Geblinger D, Geiger B, Addadi L (2009) Surface-induced regulation of podosome organization and dynamics in cultured osteoclasts. Chem Bio Chem 10(1):158–165
Gallop JL, McMahon HT (2005) BAR domains and membrane curvature: bringing your curves to the BAR. Biochem Soc Symp 72:223–231
Tsuboi S, Takada H, Hara T, Mochizuki N, Funyu T, Saitoh H, Terayama Y, Yamaya K, Ohyama C, Nonoyama S, Ochs HD (2009) FBP17 mediates a common molecular step in the formation of podosomes and phagocytic cups in macrophages. J Biol Chem 284(13):8548–8556
Nusblat LM, Dovas A, Cox D (2011) The non-redundant role of N-WASP in podosome-mediated matrix degradation in macrophages. Eur J Cell Biol 90(2–3):205–212
West MA, Prescott AR, Chan KM, Zhou Z, Rose-John S, Scheller J, Watts C (2008) TLR ligand-induced podosome disassembly in dendritic cells is ADAM17 dependent. J Cell Biol 182(5):993–1005
Goto T, Maeda H, Tanaka T (2002) A selective inhibitor of matrix metalloproteinases inhibits the migration of isolated osteoclasts by increasing the life span of podosomes. J Bone Miner Metab 20:98–105
Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Génot E (2006) Transforming growth factor β induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol 26(9):3582–3594
Dovas A, Gevrey JC, Grossi A, Park H, Abou-Kheir W, Cox D (2009) Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci 122(21):3873–3882
Burgdorf S, Lukacs-Kornek V, Kurts C (2006) The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol 176(11):6770–6776
Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y (2000) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100(5):587–597
McMichael BK, Cheney RE, Lee BS (2010) Myosin X regulates sealing zone patterning in osteoclasts through linkage of podosomes and microtubules. J Biol Chem 285:9506–9515
Cougoule C, Carreno S, Castandet J, Labrousse A, Astarie-Dequeker C, Poincloux R, Le Cabec V, Maridonneau-Parini I (2005) Activation of the lysosome associated p61Hck isoform triggers the biogenesis of podosomes. Traffic 6:682–694
Wiesner C, El Azzouzi K, Linder S (2013) A specific subset of RabGTPases controls cell surface exposure of MT1-MMP, extracellular matrix degradation and three-dimensional invasion of macrophages. J Cell Sci 126(Pt13):2820–2833
Sousa AD, Cheney RE (2005) Myosin-X: a molecular motor at the cell’s fingertips. Trends Cell Biol 15(10):533–539
Purev E, Neff L, Horne WC, Baron R (2009) c-Cbl and Cbl-b act redundantly to protect osteoclasts from apoptosis and to displace HDAC6 from β-tubulin, stabilizing microtubules and podosomes. Mol Biol Cell 20(18):4021–4030
McMichael BK, Scherer KF, Franklin NC, Lee BS (2014) The RhoGAP activity of myosin IXB is critical for osteoclast podosome patterning, motility, and resorptive capacity. PLoS One 9(1):e87402
Verhey KJ, Hammond JW (2009) Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10(11):765–777
Sirajuddin M, Rice LM, Vale RD (2014) Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16(4):335–344
Bhuwania R, Castro-Castro A, Linder S (2014) Microtubule acetylation regulates dynamics of KIF1C-powered vesicles and contact of microtubule plus ends with podosomes. Eur. J. Cell. Biol. doi:10.1016/j.ejcb.2014.07.006 (in press)
Friedl P, Zänker KS, Bröcker EB (1998) Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech 43(5):369–378
Wiesner C, Le-Cabec V, El Azzouzi K, Maridonneau-Parini I, Linder S (2014) Podosomes in space: Macrophage migration and matrix degradation in 2D and 3D settings. Cell Adh Migr 8(3)
Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201(7):1069–1084
Sabeh F, Shimizu-Hirota R, Weiss SJ (2009) Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol 185:11–19
Gui P, Labrousse A, Van Goethem E, Besson A, Maridonneau-Parini I, Le Cabec V (2014) Rho/ROCK pathway inhibition by CDK inhibitor p27kip1 participates in the onset of macrophage 3D-mesenchymal migration. J Cell Sci (pii: jcs.150987)
Guiet R, Vérollet C, Lamsoul I, Cougoule C, Poincloux R, Labrousse A, Calderwood DA, Glogauer M, Lutz PG, Maridonneau-Parini I (2012) Macrophage mesenchymal migration requires podosome stabilization by filamin A. J Biol Chem 287(16):13051–13062
van Goethem E, Guiet R, Balor S, Charrière GM, Poincloux R, Labrousse A, Maridonneau-Parini I, Le Cabec V (2011) Macrophage podosomes go 3D. Eur J Cell Biol 90(2–3):224–236
Jevnikar Z, Mirković B, Fonović UP, Zidar N, Švajger U, Kos J (2012) Three-dimensional invasion of macrophages is mediated by cysteine cathepsins in protrusive podosomes. Eur J Immunol 42(12):3429–3441
Snapper SB, Meelu P, Nguyen D, Stockton BM, Bozza P, Alt FW, Rosen FS, von Andrian UH, Klein C (2005) WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J Leukoc Biol 77(6):993–998
Thrasher AJ, Burns S, Lorenzi R, Jones GE (2000) TheWiskott-Aldrich syndrome: disordered actin dynamics in haematopoietic cells. Immunol Rev 178:118–128
Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, Chow J, Chambers T, Thrasher AJ (2004) WASP deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood 103(9):3552–3561
Soriano P, Montgomery C, Geske R, Bradley A (1991) Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64(4):693–702
Vérollet C, Gallois A, Dacquin R, Lastrucci C, Pandruvada SN, Ortega N, Poincloux R, Behar A, Cougoule C, Lowell C, Al Saati T, Jurdic P, Maridonneau-Parini I (2013) Hck contributes to bone homeostasis by controlling the recruitment of osteoclast precursors. FASEB J 27(9):3608–3618
Kilic SS, Etzioni A (2009) The clinical spectrum of leukocyte adhesion deficiency (LAD) III due to defective CalDAG-GEF1. J Clin Immunol 29(1):117–122
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196(3):254–265
Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, Jones JG (2009) Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res 15(7):2433–2441
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2):263–266
Guiet R, Van Goethem E, Cougoule C, Balor S, Valette A, Al Saati T, Lowell CA, Le Cabec V, Maridonneau-Parini I (2011) The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. J Immunol 187(7):3806–3814
Acknowledgments
We thank Koen van den Dries for contributing images and discussions. We apologize to all authors whose work was not mentioned owing to space limitations. Current research on podosomes in the SL lab has received funding from Deutsche Forschungsgemeinschaft (LI925/2-2, LI925/3-2) and the Wilhelm Sander-Stiftung (2012.026.1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linder, S., Wiesner, C. Tools of the trade: podosomes as multipurpose organelles of monocytic cells. Cell. Mol. Life Sci. 72, 121–135 (2015). https://doi.org/10.1007/s00018-014-1731-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1731-z