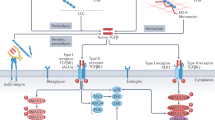
Abstract
The ability of cardiomyocytes to detect mechanical and humoral stimuli is critical for adaptation of the myocardium in response to new conditions and for sustaining the increased workload during stress. While certain stimuli mediate a beneficial adaptation to stress conditions, others result in maladaptive remodelling, ultimately leading to heart failure. Specific signalling pathways activating either adaptive or maladaptive cardiac remodelling have been identified. Paradoxically, however, in a number of cases, the transduction pathways involved in such opposing responses engage the same signalling proteins. A notable example is the Raf–MEK1/2–ERK1/2 signalling pathway that can control both adaptive and maladaptive remodelling. ERK1/2 signalling requires a signalosome complex where a scaffold protein drives the assembly of these three kinases into a linear pathway to facilitate their sequential phosphorylation, ultimately targeting specific effector molecules. Interestingly, a number of different Raf–MEK1/2–ERK1/2 scaffold proteins have been identified, and their role in determining the adaptive or maladaptive cardiac remodelling is a promising field of investigation for the development of therapeutic strategies capable of selectively potentiating the adaptive response.


Similar content being viewed by others
References
Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G (2004) Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc Res 63(3):373–380. doi:10.1016/j.cardiores.2004.04.031
Frey N, Olson EN (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65:45–79. doi:10.1146/annurev.physiol.65.092101.142243
McMullen JR, Jennings GL (2007) Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34(4):255–262. doi:10.1111/j.1440-1681.2007.04585.x
Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW 2nd (1998) Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation 97(15):1488–1495
Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA (2002) Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 105(1):85–92
Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J Jr (2002) Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 99(19):12333–12338. doi:10.1073/pnas.172376399
Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S (2000) The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J 19(11):2537–2548. doi:10.1093/emboj/19.11.2537
Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A (2002) Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277(25):22896–22901. doi:10.1074/jbc.M200347200
Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM (2002) Regulation of myocardial contractility and cell size by distinct PI3 K-PTEN signaling pathways. Cell 110(6):737–749. doi:S0092867402009698
Rose BA, Force T, Wang Y (2010) Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev 90(4):1507–1546. doi:10.1152/physrev.00054.2009
Lorenz K, Schmitt JP, Vidal M, Lohse MJ (2009) Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol 41(12):2351–2355. doi:10.1016/j.biocel.2009.08.002
Casar B, Pinto A, Crespo P (2009) ERK dimers and scaffold proteins: unexpected partners for a forgotten (cytoplasmic) task. Cell Cycle 8(7):1007–1013. doi:10.4161/cc.8.7.8078
Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarini M, Hori M, Otsu K (2004) Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest 114(7):937–943. doi:10.1172/JCI20317
Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ (2004) Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation 110(6):718–723. doi:10.1161/01.CIR.0000138190.50127.6A
Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19(23):6341–6350. doi:10.1093/emboj/19.23.6341
Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD (2007) Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA 104(35):14074–14079. doi:10.1073/pnas.0610906104
Hunter JJ, Tanaka N, Rockman HA, Ross J Jr, Chien KR (1995) Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem 270(39):23173–23178
Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, Chen J, Ross J Jr, Chien KR, Lederer JW, Wang Y (2004) Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol 286(1):H424–H433. doi:10.1152/ajpheart.00110.2003
Kehat I, Molkentin JD (2010) Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann NY Acad Sci 1188:96–102. doi:10.1111/j.1749-6632.2009.05088.x
Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y (2008) The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat 29(8):992–1006. doi:10.1002/humu.20748
Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, Lopez Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD (2007) Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet 39(8):1007–1012. doi:10.1038/ng2073
Nakamura T, Colbert M, Krenz M, Molkentin JD, Hahn HS, Dorn GW 2nd, Robbins J (2007) Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest 117(8):2123–2132. doi:10.1172/JCI30756
Bogoyevitch MA, Sugden PH (1996) The role of protein kinases in adaptational growth of the heart. Int J Biochem Cell Biol 28(1):1–12. doi:1357272595001425
Bueno OF, Molkentin JD (2002) Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res 91(9):776–781
Dorn GW 2nd, Force T (2005) Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 115(3):527–537. doi:10.1172/JCI24178
Wu X, Simpson J, Hong JH, Kim KH, Thavarajah NK, Backx PH, Neel BG, Araki T (2011) MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1 (L613 V) mutation. J Clin Invest 121(3):1009–1025. doi:10.1172/JCI44929
Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, Bonne G, Worman HJ (2007) Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest 117(5):1282–1293. doi:10.1172/JCI29042
Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ (2009) Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet 18(2):241–247. doi:10.1093/hmg/ddn343
Wu W, Muchir A, Shan J, Bonne G, Worman HJ (2011) Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene. Circulation 123(1):53–61. doi:CIRCULATIONAHA.110.970673
Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ (2009) A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med 15(1):75–83. doi:10.1038/nm.1893
Kolch W (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6(11):827–837. doi:nrm1743
Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP (2007) Scaffold proteins of MAP-kinase modules. Oncogene 26(22):3185–3202. doi:10.1038/sj.onc.1210411
Kelly PA, Rahmani Z (2005) DYRK1A enhances the mitogen-activated protein kinase cascade in PC12 cells by forming a complex with Ras, B-Raf, and MEK1. Mol Biol Cell 16(8):3562–3573. doi:10.1091/mbc.E04-12-1085
Good MC, Zalatan JG, Lim WA (2011) Scaffold proteins: hubs for controlling the flow of cellular information. Science 332(6030):680–686. doi:10.1126/science.1198701
Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308(5721):512–517. doi:10.1126/science.1109237
Tilley DG (2011) G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function. Circ Res 109(2):217–230. doi:10.1161/CIRCRESAHA.110.231225
Vidal M, Wieland T, Lohse MJ, Lorenz K (2012) Beta-adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway. Cardiovasc Res. doi:10.1093/cvr/cvs249
Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA (2001) Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation 103(10):1453–1458
Belcheva MM, Coscia CJ (2002) Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals 11(1):34–44. doi:10.1159/000057320
Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW 2nd, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J (2008) An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest 118(12):3870–3880. doi:10.1172/JCI34472
Patel PA, Tilley DG, Rockman HA (2008) Beta-arrestin-mediated signaling in the heart. Circ J 72(11):1725–1729. doi:JST.JSTAGE/circj/CJ-08-0734
Rajagopal S, Rajagopal K, Lefkowitz RJ (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9(5):373–386. doi:10.1038/nrd3024
Noor N, Patel CB, Rockman HA (2011) Beta-arrestin: a signaling molecule and potential therapeutic target for heart failure. J Mol Cell Cardiol 51(4):534–541. doi:10.1016/j.yjmcc.2010.11.005
Cowling BS, Cottle DL, Wilding BR, D’Arcy CE, Mitchell CA, McGrath MJ (2011) Four and a half LIM protein 1 gene mutations cause four distinct human myopathies: a comprehensive review of the clinical, histological and pathological features. Neuromuscul Disord 21(4):237–251. doi:10.1016/j.nmd.2011.01.001
Lee SM, Tsui SK, Chan KK, Garcia-Barcelo M, Waye MM, Fung KP, Liew CC, Lee CY (1998) Chromosomal mapping, tissue distribution and cDNA sequence of four-and-a-half LIM domain protein 1 (FHL1). Gene 216(1):163–170. doi:S0378-1119(98)00302-3
Friedrich FW, Wilding BR, Reischmann S, Crocini C, Lang P, Charron P, Muller OJ, McGrath MJ, Vollert I, Hansen A, Linke WA, Hengstenberg C, Bonne G, Morner S, Wichter T, Madeira H, Arbustini E, Eschenhagen T, Mitchell CA, Isnard R, Carrier L (2012) Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum Mol Genet 21(14):3237–3254. doi:10.1093/hmg/dds157
Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J (2000) Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev 95(1–2):259–265. doi:S0925477300003415
Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE (2003) Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation 108(23):2926–2933. doi:10.1161/01.CIR.0000101922.18151.7B
Hwang DM, Dempsey AA, Wang RX, Rezvani M, Barrans JD, Dai KS, Wang HY, Ma H, Cukerman E, Liu YQ, Gu JR, Zhang JH, Tsui SK, Waye MM, Fung KP, Lee CY, Liew CC (1997) A genome-based resource for molecular cardiovascular medicine: toward a compendium of cardiovascular genes. Circulation 96(12):4146–4203
Hwang DM, Dempsey AA, Lee CY, Liew CC (2000) Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics 66(1):1–14. doi:10.1006/geno.2000.6171
Lim DS, Roberts R, Marian AJ (2001) Expression profiling of cardiac genes in human hypertrophic cardiomyopathy: insight into the pathogenesis of phenotypes. J Am Coll Cardiol 38(4):1175–1180. doi:S0735-1097(01)01509-1
Raskin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, Yamazaki KG, Granzier HL, Gregorio CC, McCulloch AD, Omens JH, Sheikh F (2012) A novel mechanism involving four-and-a-half lim domain protein-1 and extracellular signal-regulated kinase-2 regulates titin phosphorylation and mechanics. J Biol Chem 287(35):29273–29284. doi:10.1074/jbc.M112.372839
Ren JG, Li Z, Sacks DB (2007) IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci USA 104(25):10465–10469. doi:10.1073/pnas.0611308104
Roy M, Li Z, Sacks DB (2005) IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol 25(18):7940–7952. doi:10.1128/MCB.25.18.7940-7952.2005
Sbroggio M, Carnevale D, Bertero A, Cifelli G, De Blasio E, Mascio G, Hirsch E, Bahou WF, Turco E, Silengo L, Brancaccio M, Lembo G, Tarone G (2011) IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload. Cardiovasc Res 91(3):456–464. doi:10.1093/cvr/cvr103
Chen F, Zhu HH, Zhou LF, Wu SS, Wang J, Chen Z (2010) IQGAP1 is overexpressed in hepatocellular carcinoma and promotes cell proliferation by Akt activation. Exp Mol Med 42(7):477–483. doi:10.3858/emm.2010.42.7.049
Sbroggio M, Bertero A, Velasco S, Fusella F, De Blasio E, Bahou WF, Silengo L, Turco E, Brancaccio M, Tarone G (2011) ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1. J Cell Sci 124(Pt 20):3515–3524. doi:10.1242/jcs.091140
Ferretti R, Sbroggio M, Di Savino A, Fusella F, Bertero A, Michowski W, Tarone G, Brancaccio M (2011) Morgana and Melusin: Two fairies chaperoning signal transduction. Cell Cycle 10(21):3678–3683. doi:18202
Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11(7):515–528. doi:10.1038/nrm2918
Morrison DK, Davis RJ (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19:91–118. doi:10.1146/annurev.cellbio.19.111401.091942
Muller J, Ory S, Copeland T, Piwnica-Worms H, Morrison DK (2001) C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell 8(5):983–993. doi:S1097-2765(01)00383-5
Matheny SA, Chen C, Kortum RL, Razidlo GL, Lewis RE, White MA (2004) Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature 427(6971):256–260. doi:10.1038/nature02237
Cacace AM, Michaud NR, Therrien M, Mathes K, Copeland T, Rubin GM, Morrison DK (1999) Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14–3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol 19(1):229–240
Kortum RL, Johnson HJ, Costanzo DL, Volle DJ, Razidlo GL, Fusello AM, Shaw AS, Lewis RE (2006) The molecular scaffold kinase suppressor of Ras 1 is a modifier of RasV12-induced and replicative senescence. Mol Cell Biol 26(6):2202–2214. doi:10.1128/MCB.26.6.2202-2214.2006
Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE (2005) The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol Cell Biol 25(17):7592–7604. doi:10.1128/MCB.25.17.7592-7604.2005
Zhang Y, Li X, Carpinteiro A, Goettel JA, Soddemann M, Gulbins E (2011) Kinase suppressor of Ras-1 protects against pulmonary Pseudomonas aeruginosa infections. Nat Med 17(3):341–346. doi:10.1038/nm.2296
Vomastek T, Schaeffer HJ, Tarcsafalvi A, Smolkin ME, Bissonette EA, Weber MJ (2004) Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc Natl Acad Sci USA 101(18):6981–6986. doi:10.1073/pnas.0305894101
Ishibe S, Joly D, Liu ZX, Cantley LG (2004) Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell 16(2):257–267. doi:10.1016/j.molcel.2004.10.006
Yin G, Haendeler J, Yan C, Berk BC (2004) GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol 24(2):875–885
Pang J, Xu X, Getman MR, Shi X, Belmonte SL, Michaloski H, Mohan A, Blaxall BC, Berk BC (2011) G protein coupled receptor kinase 2 interacting protein 1 (GIT1) is a novel regulator of mitochondrial biogenesis in heart. J Mol Cell Cardiol 51(5):769–776. doi:10.1016/j.yjmcc.2011.06.020
Himpel S, Tegge W, Frank R, Leder S, Joost HG, Becker W (2000) Specificity determinants of substrate recognition by the protein kinase DYRK1A. J Biol Chem 275(4):2431–2438
Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A (2006) A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441(7093):646–650. doi:10.1038/nature04631
Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, Yamasaki N, Miyakawa T, Francke U, Graef IA, Crabtree GR (2006) NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 441(7093):595–600. doi:10.1038/nature04678
Kuhn C, Frank D, Will R, Jaschinski C, Frauen R, Katus HA, Frey N (2009) DYRK1A is a novel negative regulator of cardiomyocyte hypertrophy. J Biol Chem 284(25):17320–17327. doi:10.1074/jbc.M109.006759
da Costa Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, De Windt LJ (2010) MicroRNA-199b targets the nuclear kinase DYRK1A in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol 12(12):1220–1227. doi:10.1038/ncb2126
Grebe C, Klingebiel TM, Grau SP, Toischer K, Didie M, Jacobshagen C, Dullin C, Hasenfuss G, Seidler T (2011) Enhanced expression of DYRK1A in cardiomyocytes inhibits acute NFAT activation but does not prevent hypertrophy in vivo. Cardiovasc Res 90(3):521–528. doi:10.1093/cvr/cvr023
Calvo F, Agudo-Ibanez L, Crespo P (2010) The Ras-ERK pathway: understanding site-specific signaling provides hope of new anti-tumor therapies. BioEssays 32(5):412–421. doi:10.1002/bies.200900155
Matallanas D, Crespo P (2010) New druggable targets in the Ras pathway? Curr Opin Mol Ther 12(6):674–683
Acknowledgments
We wish to thank R. Srinivasan for manuscript revision. This work was supported by grants from the Regione Piemonte POR F.E.S.R.2007/2013 “DRUIDI: Piattaforme Innovative per le Scienze della Vita” to G.T. and M.B., Telethon GGP12047 to G.T., FIRB RBFR10L0GK to M.S., PRIN 2010J8RYS7 to M.B. and PRIN 2010RNXM9C to G.T.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarone, G., Sbroggiò, M. & Brancaccio, M. Key role of ERK1/2 molecular scaffolds in heart pathology. Cell. Mol. Life Sci. 70, 4047–4054 (2013). https://doi.org/10.1007/s00018-013-1321-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1321-5