Abstract
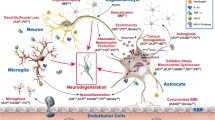
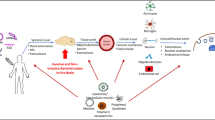
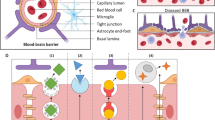
RNA interference has been envisaged as a powerful tool for molecular and clinical investigation with a great potential for clinical applications. In recent years, increased understanding of cancer biology and stem cell biology has dramatically accelerated the development of technology for cell and gene therapy in these areas. This paper is a review of the most recent report of innovative use of siRNA to benefit several central nervous system diseases. Furthermore, a description is made of innovative strategies of delivery into the brain by means of viral and non-viral vectors with high potential for translation into clinical use. Problems are also highlighted that might hamper the transition from bench to bed, analyzing the lack of reliable preclinical models with predictive validity and the lack of effective delivery systems, which are able to overcome biological barriers and specifically reach the brain site of action.

Similar content being viewed by others
References
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811. doi:10.1038/35888
Zhuo M (2011) Cortical plasticity as a new endpoint measurement for chronic pain. Mol Pain 7:54. doi:10.1186/1744-8069-7-54
Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J, Pourabdollah-Nejad DS, Zhang C, Zhang L, Jiang H, Zhang ZG, Chopp M (2010) Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS ONE 5(2):e9027. doi:10.1371/journal.pone.0009027
Sayed D, Abdellatif M (2011) MicroRNAs in development and disease. Physiol Rev 91(3):827–887. doi:10.1152/physrev.00006.2010
Ahlenstiel CL, Lim HG, Cooper DA, Ishida T, Kelleher AD, Suzuki K (2012) Direct evidence of nuclear argonaute distribution during transcriptional silencing links the actin cytoskeleton to nuclear RNAi machinery in human cells. Nucleic Acids Res 40(4):1579–1595. doi:10.1093/nar/gkr891
Liu J, Hu J, Corey DR (2012) Expanding the action of duplex RNAs into the nucleus: redirecting alternative splicing. Nucleic Acids Res 40(3):1240–1250. doi:10.1093/nar/gkr780
Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, Tuschl T (2003) Sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing. Antisense Nucleic Acid Drug Dev 13(2):83–105. doi:10.1089/108729003321629638
Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP (2004) Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432(7014):173–178. doi:10.1038/nature03121
Rao DD, Senzer N, Cleary MA, Nemunaitis J (2009) Comparative assessment of siRNA and shRNA off target effects: what is slowing clinical development. Cancer Gene Ther 16(11):807–809. doi:10.1038/cgt.2009.53
Fluiter K, Mook OR, Baas F (2009) The therapeutic potential of LNA-modified siRNAs: reduction of off-target effects by chemical modification of the siRNA sequence. Methods Mol Biol 487:189–203. doi:10.1007/978-1-60327-547-7_9
Ghafouri-Fard S (2012) siRNA and cancer immunotherapy. Immunotherapy 4(9):907–917. doi:10.2217/imt.12.87
Nolte A, Ott K, Rohayem J, Walker T, Schlensak C, Wendel HP (2012) Modification of small interfering RNAs to prevent off-target effects by the sense strand. N Biotechnol. doi:10.1016/j.nbt.2012.10.001
Gouda N, Miyata K, Christie RJ, Suma T, Kishimura A, Fukushima S, Nomoto T, Liu X, Nishiyama N, Kataoka K (2013) Silica nanogelling of environment-responsive PEGylated polyplexes for enhanced stability and intracellular delivery of siRNA. Biomaterials 34(2):562–570. doi:10.1016/j.biomaterials.2012.09.077
Fountaine TM, Wood MJ, Wade-Martins R (2005) Delivering RNA interference to the mammalian brain. Curr Gene Ther 5(4):399–410
Perez-Martinez FC, Guerra J, Posadas I, Cena V (2011) Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm Res 28(8):1843–1858. doi:10.1007/s11095-010-0364-7
Boudreau RL, Rodriguez-Lebron E, Davidson BL (2011) RNAi medicine for the brain: progresses and challenges. Hum Mol Genet 20 (R1):R21–R27. doi:10.1093/hmg/ddr137
Boudreau RL, Spengler RM, Davidson BL (2011) Rational design of therapeutic siRNAs: minimizing off-targeting potential to improve the safety of RNAi therapy for Huntington’s disease. Mol Ther 19(12):2169–2177. doi:10.1038/mt.2011.185
Kleinman ME, Kaneko H, Cho WG, Dridi S, Fowler BJ, Blandford AD, Albuquerque RJ, Hirano Y, Terasaki H, Kondo M, Fujita T, Ambati BK, Tarallo V, Gelfand BD, Bogdanovich S, Baffi JZ, Ambati J (2012) Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol Ther 20(1):101–108. doi:10.1038/mt.2011.212
Xia H, Mao Q, Paulson HL, Davidson BL (2002) siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol 20(10):1006–1010
Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL (2004) RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 10(8):816–820. doi:10.1038/nm1076
Waehler R, Russell SJ, Curiel DT (2007) Engineering targeted viral vectors for gene therapy. Nat Rev Genet 8(8):573–587
Schagen FH, Ossevoort M, Toes RE, Hoeben RC (2004) Immune responses against adenoviral vectors and their transgene products: a review of strategies for evasion. Crit Rev Oncol Hematol 50(1):51–70. doi:10.1016/S1040-8428(03)00172-0
Zhang Y, Friedlander RM (2011) Using non-coding small RNAs to develop therapies for Huntington’s disease. Gene Ther 18(12):1139–1149. doi:10.1038/gt.2011.170
Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA (2006) Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441(7092):537–541. doi:10.1038/nature04791
Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ (2009) Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73(20):1662–1669. doi:10.1212/WNL.0b013e3181c29356
Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ (2008) Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 70(21):1980–1983. doi:10.1212/01.wnl.0000312381.29287.ff
Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS (2010) Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther 18(8):1458–1461. doi:10.1038/mt.2010.106
LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Van Meter L, Sapan CV, During MJ, Kaplitt MG, Feigin A (2011) AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 10(4):309–319. doi:10.1016/S1474-4422(11)70039-4
Souweidane MM, Fraser JF, Arkin LM, Sondhi D, Hackett NR, Kaminsky SM, Heier L, Kosofsky BE, Worgall S, Crystal RG, Kaplitt MG (2010) Gene therapy for late infantile neuronal ceroid lipofuscinosis: neurosurgical considerations. J Neurosurg Pediatr 6(2):115–122. doi:10.3171/2010.4.PEDS09507
Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG (2008) Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther 19(5):463–474. doi:10.1089/hum.2008.022
Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N (2007) Transvascular delivery of small interfering RNA to the central nervous system. Nature 448(7149):39–43. doi:10.1038/nature05901
Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, Castaigne JP, Beliveau R (2008) Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 324(3):1064–1072. doi:10.1124/jpet.107.131318
Al-Jamal KT, Gherardini L, Bardi G, Nunes A, Guo C, Bussy C, Herrero MA, Bianco A, Prato M, Kostarelos K, Pizzorusso T (2011) Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci USA 108(27):10952–10957. doi:10.1073/pnas.1100930108
Bonoiu AC, Bergey EJ, Ding H, Hu R, Kumar R, Yong KT, Prasad PN, Mahajan S, Picchione KE, Bhattacharjee A, Ignatowski TA (2011) Gold nanorod—siRNA induces efficient in vivo gene silencing in the rat hippocampus. Nanomedicine (Lond) 6(4):617–630. doi:10.2217/nnm.11.20
Bonoiu AC, Mahajan SD, Ding H, Roy I, Yong KT, Kumar R, Hu R, Bergey EJ, Schwartz SA, Prasad PN (2009) Nanotechnology approach for drug addiction therapy: gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc Natl Acad Sci USA 106(14):5546–5550
Posadas I, Guerra FJ, Cena V (2010) Nonviral vectors for the delivery of small interfering RNAs to the CNS. Nanomedicine (Lond) 5(8):1219–1236. doi:10.2217/nnm.10.105
Liang Y, Liu Z, Shuai X, Wang W, Liu J, Bi W, Wang C, Jing X, Liu Y, Tao E (2012) Delivery of cationic polymer-siRNA nanoparticles for gene therapies in neural regeneration. Biochem Biophys Res Commun 421(4):690–695. doi:10.1016/j.bbrc.2012.03.155
Lares MR, Rossi JJ, Ouellet DL (2010) RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol 28(11):570–579. doi:10.1016/j.tibtech.2010.07.009
Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM (2012) Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med 4 (130):130ra146. doi:10.1126/scitranslmed.3003162
Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, Lou J, Jiang C (2009) Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials 30(25):4195–4202. doi:10.1016/j.biomaterials.2009.02.051
Tosi G, Costantino L, Ruozi B, Forni F, Vandelli MA (2008) Polymeric nanoparticles for the drug delivery to the central nervous system. Expert Opin Drug Deliv 5(2):155–174. doi:10.1517/17425247.5.2.155
Li S (1999) Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res 48(3):342–353. doi:10.1002/(SICI)1097-4636(1999)48:3<342:AID-JBM20>3.0.CO;2-7
Bazile DV, Ropert C, Huve P, Verrecchia T, Marlard M, Frydman A, Veillard M, Spenlehauer G (1992) Body distribution of fully biodegradable [14C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials 13(15):1093–1102
Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9(8):615–627. doi:10.1038/nrd2591
Jokerst JV, Lobovkina T, Zare RN, Gambhir SS (2011) Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 6(4):715–728. doi:10.2217/nnm.11.19
Li W, Szoka FC Jr (2007) Lipid-based nanoparticles for nucleic acid delivery. Pharm Res 24(3):438–449. doi:10.1007/s11095-006-9180-5
Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG (2010) Lipid-based nanotherapeutics for siRNA delivery. J Intern Med 267(1):9–21. doi:10.1111/j.1365-2796.2009.02189.x
Wu SY, McMillan NA (2009) Lipidic systems for in vivo siRNA delivery. AAPS J 11(4):639–652. doi:10.1208/s12248-009-9140-1
Pardridge WM (2007) shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev 59(2–3):141–152. doi:10.1016/j.addr.2007.03.008
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. doi:10.1038/nbt.1807
van den Boorn JG, Schlee M, Coch C, Hartmann G (2011) SiRNA delivery with exosome nanoparticles. Nat Biotechnol 29(4):325–326. doi:10.1038/nbt.1830
Nakajima H, Kubo T, Semi Y, Itakura M, Kuwamura M, Izawa T, Azuma YT, Takeuchi T (2012) A rapid, targeted, neuron-selective, in vivo knockdown following a single intracerebroventricular injection of a novel chemically modified siRNA in the adult rat brain. J Biotechnol 157(2):326–333. doi:10.1016/j.jbiotec.2011.10.003
Gupta AK, Eshraghi Y, Gliniak C, Gosain AK (2010) Nonviral transfection of mouse calvarial organ in vitro using Accell-modified siRNA. Plast Reconstr Surg 125(2):494–501. doi:10.1097/PRS.0b013e3181c82df1
Larsen HO, Roug AS, Nielsen K, Sondergaard CS, Hokland P (2011) Nonviral transfection of leukemic primary cells and cells lines by siRNA-a direct comparison between Nucleofection and Accell delivery. Exp Hematol 39(11):1081–1089. doi:10.1016/j.exphem.2011.08.003
Hanson LR, Frey WH 2nd (2008) Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 9(Suppl 3):S5. doi:10.1186/1471-2202-9-S3-S5
Wen MM (2011) Olfactory targeting through intranasal delivery of biopharmaceutical drugs to the brain: current development. Discov Med 11(61):497–503
Capsoni S, Covaceuszach S, Ugolini G, Spirito F, Vignone D, Stefanini B, Amato G, Cattaneo A (2009) Delivery of NGF to the brain: intranasal versus ocular administration in anti-NGF transgenic mice. J Alzheimers Dis 16(2):371–388. doi:10.3233/JAD-2009-0953
Capsoni S, Marinelli S, Ceci M, Vignone D, Amato G, Malerba F, Paoletti F, Meli G, Viegi A, Pavone F, Cattaneo A (2012) Intranasal “painless” human nerve growth factors slows amyloid neurodegeneration and prevents memory deficits in App X PS1 mice. PLoS ONE 7(5):e37555. doi:10.1371/journal.pone.0037555
Mast TG, Fadool DA (2012) Mature and precursor brain-derived neurotrophic factor have individual roles in the mouse olfactory bulb. PLoS ONE 7(2):e31978. doi:10.1371/journal.pone.0031978
Vaka SR, Murthy SN, Balaji A, Repka MA (2012) Delivery of brain-derived neurotrophic factor via nose-to-brain pathway. Pharm Res 29(2):441–447. doi:10.1007/s11095-011-0572-9
Farah MH (2007) RNAi silencing in mouse models of neurodegenerative diseases. Curr Drug Deliv 4(2):161–167
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69(1):29–38. doi:10.1001/archneurol.2011.233
Renner DB, Frey WH 2nd, Hanson LR (2012) Intranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathway. Neurosci Lett 513(2):193–197. doi:10.1016/j.neulet.2012.02.037
Perez AP, Mundina-Weilenmann C, Romero EL, Morilla MJ (2012) Increased brain radioactivity by intranasal P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int J Nanomed 7:1373–1385. doi:10.2147/IJN.S28261
Kim ID, Lim CM, Kim JB, Nam HY, Nam K, Kim SW, Park JS, Lee JK (2010) Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J Control Release 142(3):422–430. doi:10.1016/j.jconrel.2009.11.011
Koutsilieri E, Rethwilm A, Scheller C (2007) The therapeutic potential of siRNA in gene therapy of neurodegenerative disorders. J Neural Transm Suppl 72:43–49
Nilsson P, Iwata N, Muramatsu S, Tjernberg LO, Winblad B, Saido TC (2010) Gene therapy in Alzheimer’s disease—potential for disease modification. J Cell Mol Med 14(4):741–757. doi:10.1111/j.1582-4934.2010.01038.x
Lovett-Racke AE, Cravens PD, Gocke AR, Racke MK, Stuve O (2005) Therapeutic potential of small interfering RNA for central nervous system diseases. Arch Neurol 62(12):1810–1813. doi:10.1001/archneur.62.12.1810
Rodriguez-Lebron E, Gonzalez-Alegre P (2006) Silencing neurodegenerative disease: bringing RNA interference to the clinic. Expert Rev Neurother 6(2):223–233. doi:10.1586/14737175.6.2.223
Orlacchio A, Bernardi G, Orlacchio A, Martino S (2007) RNA interference as a tool for Alzheimer’s disease therapy. Mini Rev Med Chem 7(11):1166–1176
Maxwell MM (2009) RNAi applications in therapy development for neurodegenerative disease. Curr Pharm Des 15(34):3977–3991
Acquatella-Tran Van Ba I, Marchal S, Francois F, Silhol M, Lleres C, Michel B, Benyamin Y, Verdier JM, Trousse F, Marcilhac A (2012) Regenerating islet-derived 1alpha (Reg-1alpha) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin tumor-like 3 (EXTL3) receptor. J Biol Chem 287(7):4726–4739. doi:10.1074/jbc.M111.260349
Frykman S, Teranishi Y, Hur JY, Sandebring A, Goto Yamamoto N, Ancarcrona M, Nishimura T, Winblad B, Bogdanovic N, Schedin-Weiss S, Kihara T, Tjernberg LO (2012) Identification of two novel synaptic gamma-secretase associated proteins that affect amyloid beta-peptide levels without altering Notch processing. Neurochem Int 61(1):108–118. doi:10.1016/j.neuint.2012.03.016
Marwarha G, Dasari B, Ghribi O (2012) Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell Signal 24(2):484–492. doi:10.1016/j.cellsig.2011.09.029
Nawrot B (2004) Targeting BACE with small inhibitory nucleic acids—a future for Alzheimer’s disease therapy? Acta Biochim Pol 51(2):431–444. doi:035001431
Ohno M (2006) Genetic and pharmacological basis for therapeutic inhibition of beta- and gamma-secretases in mouse models of Alzheimer’s memory deficits. Rev Neurosci 17(4):429–454
Peng KA, Masliah E (2010) Lentivirus-expressed siRNA vectors against Alzheimer disease. Methods Mol Biol 614:215–224. doi:10.1007/978-1-60761-533-0_15
Prasanthi JR, Larson T, Schommer J, Ghribi O (2011) Silencing GADD153/CHOP gene expression protects against Alzheimer’s disease-like pathology induced by 27-hydroxycholesterol in rabbit hippocampus. PLoS ONE 6(10):e26420. doi:10.1371/journal.pone.0026420
Southwell AL, Patterson PH (2011) Gene therapy in mouse models of Huntington disease. Neuroscientist 17(2):153–162. doi:10.1177/1073858410386236
Reddy PH, Williams M, Charles V, Garrett L, Pike-Buchanan L, Whetsell WO Jr, Miller G, Tagle DA (1998) Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat Genet 20(2):198–202. doi:10.1038/2510
Miller JP, Hughes RE (2011) Protein interactions and target discovery in Huntington’s disease. In: Lo DC, Hughes RE (eds) Neurobiology of Huntington’s disease: applications to drug discovery. Frontiers in Neuroscience, Boca Raton (FL)
Ross CA, Shoulson I (2009) Huntington disease: pathogenesis, biomarkers, and approaches to experimental therapeutics. Parkinsonism Relat Disord 15(Suppl 3):S135–S138. doi:10.1016/S1353-8020(09)70800-4
Wang X, Sirianni A, Pei Z, Cormier K, Smith K, Jiang J, Zhou S, Wang H, Zhao R, Yano H, Kim JE, Li W, Kristal BS, Ferrante RJ, Friedlander RM (2011) The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J Neurosci 31(41):14496–14507. doi:10.1523/JNEUROSCI.3059-11.2011
Chen BS, Thomas EV, Sanz-Clemente A, Roche KW (2011) NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants. J Neurosci 31(1):89–96. doi:10.1523/JNEUROSCI.1034-10.2011
Tong Y, Ha TJ, Liu L, Nishimoto A, Reiner A, Goldowitz D (2011) Spatial and temporal requirements for huntingtin (Htt) in neuronal migration and survival during brain development. J Neurosci 31(41):14794–14799. doi:10.1523/JNEUROSCI.2774-11.2011
Lombardi MS, Jaspers L, Spronkmans C, Gellera C, Taroni F, Di Maria E, Donato SD, Kaemmerer WF (2009) A majority of Huntington’s disease patients may be treatable by individualized allele-specific RNA interference. Exp Neurol 217(2):312–319. doi:10.1016/j.expneurol.2009.03.004
Pfister EL, Kennington L, Straubhaar J, Wagh S, Liu W, DiFiglia M, Landwehrmeyer B, Vonsattel JP, Zamore PD, Aronin N (2009) Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington’s disease patients. Curr Biol 19(9):774–778. doi:10.1016/j.cub.2009.03.030
Hu J, Liu J, Corey DR (2010) Allele-selective inhibition of huntingtin expression by switching to an miRNA-like RNAi mechanism. Chem Biol 17(11):1183–1188. doi:10.1016/j.chembiol.2010.10.013
Grondin R, Kaytor MD, Ai Y, Nelson PT, Thakker DR, Heisel J, Weatherspoon MR, Blum JL, Burright EN, Zhang Z, Kaemmerer WF (2012) Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum. Brain 135(Pt 4):1197–1209. doi:10.1093/brain/awr333
McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, Davidson BL (2011) Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther 19(12):2152–2162. doi:10.1038/mt.2011.219
Stiles DK, Zhang Z, Ge P, Nelson B, Grondin R, Ai Y, Hardy P, Nelson PT, Guzaev AP, Butt MT, Charisse K, Kosovrasti V, Tchangov L, Meys M, Maier M, Nechev L, Manoharan M, Kaemmerer WF, Gwost D, Stewart GR, Gash DM, Sah DW (2012) Widespread suppression of huntingtin with convection-enhanced delivery of siRNA. Exp Neurol 233(1):463–471. doi:10.1016/j.expneurol.2011.11.020
Manfredsson FP, Lewin AS, Mandel RJ (2006) RNA knockdown as a potential therapeutic strategy in Parkinson’s disease. Gene Ther 13(6):517–524. doi:10.1038/sj.gt.3302669
Lundberg C, Bjorklund T, Carlsson T, Jakobsson J, Hantraye P, Deglon N, Kirik D (2008) Applications of lentiviral vectors for biology and gene therapy of neurological disorders. Curr Gene Ther 8(6):461–473
Porras G, Bezard E (2008) Preclinical development of gene therapy for Parkinson’s disease. Exp Neurol 209(1):72–81. doi:10.1016/j.expneurol.2007.08.003
Ardley HC, Hung CC, Robinson PA (2005) The aggravating role of the ubiquitin-proteasome system in neurodegeneration. FEBS Lett 579(3):571–576. doi:10.1016/j.febslet.2004.12.058
Liani E, Eyal A, Avraham E, Shemer R, Szargel R, Berg D, Bornemann A, Riess O, Ross CA, Rott R, Engelender S (2004) Ubiquitylation of synphilin-1 and alpha-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson’s disease. Proc Natl Acad Sci USA 101(15):5500–5505
Nagano Y, Yamashita H, Takahashi T, Kishida S, Nakamura T, Iseki E, Hattori N, Mizuno Y, Kikuchi A, Matsumoto M (2003) Siah-1 facilitates ubiquitination and degradation of synphilin-1. J Biol Chem 278(51):51504–51514
Rott R, Szargel R, Haskin J, Bandopadhyay R, Lees AJ, Shani V, Engelender S (2011) alpha-Synuclein fate is determined by USP9X-regulated monoubiquitination. Proc Natl Acad Sci USA 108(46):18666–18671. doi:10.1073/pnas.1105725108
Yacoubian TA, Slone SR, Harrington AJ, Hamamichi S, Schieltz JM, Caldwell KA, Caldwell GA, Standaert DG (2010) Differential neuroprotective effects of 14-3-3 proteins in models of Parkinson’s disease. Cell Death Dis 1:e2. doi:10.1038/cddis.2009.4
Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A (2007) Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci 27(20):5349–5362. doi:10.1523/JNEUROSCI.4107-06.2007
Latchoumycandane C, Anantharam V, Jin H, Kanthasamy A, Kanthasamy A (2011) Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCdelta in cell culture and animal models of Parkinson’s disease. Toxicol Appl Pharmacol 256(3):314–323. doi:10.1016/j.taap.2011.07.021
Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH (2010) Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol 67(12):1464–1472. doi:10.1001/archneurol.2010.198
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta 1802(1):29–44. doi:10.1016/j.bbadis.2009.08.013
Horvath L, van Marion I, Taï K, Nielsen TT, Lundberg C (2011) Knockdown of GAD67 protein levels normalizes neuronal activity in a rat model of Parkinson’s disease. J Gene Med 13(3):188–197
Dick DM, Riley B, Kendler KS (2010) Nature and nurture in neuropsychiatric genetics: where do we stand? Dialogues Clin Neurosci 12(1):7–23
Bauer M, Praschak-Rieder N, Kasper S, Willeit M (2012) Is dopamine neurotransmission altered in prodromal schizophrenia? A review of the evidence. Curr Pharm Des 18(12):1568–1579
Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (2012) Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. doi:10.1038/mp.2012.47
Vrajova M, Pekova S, Horacek J, Hoschl C (2011) The effects of siRNA-mediated RGS4 gene silencing on the whole genome transcription profile: implications for schizophrenia. Neuro Endocrinol Lett 32(3):246–252
Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM (2006) Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res 84(1):1–14. doi:10.1016/j.schres.2006.02.009
Hattori T, Shimizu S, Koyama Y, Yamada K, Kuwahara R, Kumamoto N, Matsuzaki S, Ito A, Katayama T, Tohyama M (2010) DISC1 regulates cell-cell adhesion, cell-matrix adhesion and neurite outgrowth. Mol Psychiatry 15(8):778, 798–809. doi:10.1038/mp.2010.60
Ma X, Fei E, Fu C, Ren H, Wang G (2011) Dysbindin-1, a schizophrenia-related protein, facilitates neurite outgrowth by promoting the transcriptional activity of p53. Mol Psychiatry 16(11):1105–1116. doi:10.1038/mp.2011.43
Dyck BA, Tan ML, Daya RP, Basu D, Sookram CD, Thomas N, Mishra RK (2012) Behavioral effects of non-viral mediated RNA interference of synapsin II in the medial prefrontal cortex of the rat. Schizophr Res 137(1–3):32–38. doi:10.1016/j.schres.2012.01.029
Noori-Daloii MR, Mojarrad M, Rashidi-Nezhad A, Kheirollahi M, Shahbazi A, Khaksari M, Korzebor A, Goodarzi A, Ebrahimi M, Noori-Daloii AR (2012) Use of siRNA in knocking down of dopamine receptors, a possible therapeutic option in neuropsychiatric disorders. Mol Biol Rep 39(2):2003–2010. doi:10.1007/s11033-011-0947-3
Turner BJ, Talbot K (2008) Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol 85(1):94–134. doi:10.1016/j.pneurobio.2008.01.001
Ding H, Schwarz DS, Keene A, el Affar B, Fenton L, Xia X, Shi Y, Zamore PD, Xu Z (2003) Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell 2(4):209–217
Geng CM, Ding HL (2011) Design of functional small interfering RNAs targeting amyotrophic lateral sclerosis-associated mutant alleles. Chin Med J (Engl) 124(1):106–110
Yokota T, Miyagishi M, Hino T, Matsumura R, Tasinato A, Urushitani M, Rao RV, Takahashi R, Bredesen DE, Taira K, Mizusawa H (2004) siRNA-based inhibition specific for mutant SOD1 with single nucleotide alternation in familial ALS, compared with ribozyme and DNA enzyme. Biochem Biophys Res Commun 314(1):283–291
Prudencio M, Durazo A, Whitelegge JP, Borchelt DR (2010) An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1. Hum Mol Genet 19(24):4774–4789. doi:10.1093/hmg/ddq408
Yates D (2010) Motor neuron disease: misfolded wild-type SOD1 may link sporadic and familial ALS. Nat Rev Neurol 6(12):645. doi:10.1038/nrneurol.2010.169
Xia X, Zhou H, Huang Y, Xu Z (2006) Allele-specific RNAi selectively silences mutant SOD1 and achieves significant therapeutic benefit in vivo. Neurobiol Dis 23(3):578–586. doi:10.1016/j.nbd.2006.04.019
Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M (2005) Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med 11(4):429–433. doi:10.1038/nm1205
Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P (2005) Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med 11(4):423–428. doi:10.1038/nm1207
Towne C, Setola V, Schneider BL, Aebischer P (2011) Neuroprotection by gene therapy targeting mutant SOD1 in individual pools of motor neurons does not translate into therapeutic benefit in fALS mice. Mol Ther 19(2):274–283. doi:10.1038/mt.2010.260
Rizvanov AA, Mukhamedyarov MA, Palotas A, Islamov RR (2009) Retrogradely transported siRNA silences human mutant SOD1 in spinal cord motor neurons. Exp Brain Res 195(1):1–4. doi:10.1007/s00221-009-1742-4
Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, Shui G, Tang N, Low CM, Wenk MR, Kesavapany S (2012) Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci 32(3):1020–1034. doi:10.1523/JNEUROSCI.5177-11.2012
Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ (2011) Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci 31(46):16619–16636. doi:10.1523/JNEUROSCI.1639-11.2011
Kuzma-Kozakiewicz M, Kwiecinski H (2011) New therapeutic targets for amyotrophic lateral sclerosis. Expert Opin Ther Targets 15(2):127–143. doi:10.1517/14728222.2011.542152
Hu Q, Chen C, Khatibi NH, Li L, Yang L, Wang K, Han J, Duan W, Zhang JH, Zhou C (2011) Lentivirus-mediated transfer of MMP-9 shRNA provides neuroprotection following focal ischemic brain injury in rats. Brain Res 1367:347–359. doi:10.1016/j.brainres.2010.10.002
Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, Yang L, Wang K, Chen L, Huang H, Han J, Zhang JH, Zhou C (2009) Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol 216(1):35–46. doi:10.1016/j.expneurol.2008.11.007
Liu J, Jin X, Liu KJ, Liu W (2012) Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32(9):3044–3057. doi:10.1523/JNEUROSCI.6409-11.2012
Wang L, Chopp M, Zhang RL, Zhang L, Letourneau Y, Feng YF, Jiang A, Morris DC, Zhang ZG (2009) The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 158(4):1356–1363. doi:10.1016/j.neuroscience.2008.10.064
Bakondi B, Shimada IS, Peterson BM, Spees JL (2011) SDF-1alpha secreted by human CD133-derived multipotent stromal cells promotes neural progenitor cell survival through CXCR7. Stem Cells Dev 20(6):1021–1029. doi:10.1089/scd.2010.0198
Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH (2012) Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 32(10):3462–3473. doi:10.1523/JNEUROSCI.5686-11.2012
Arumugam TV, Cheng YL, Choi Y, Choi YH, Yang S, Yun YK, Park JS, Yang DK, Thundyil J, Gelderblom M, Karamyan VT, Tang SC, Chan SL, Magnus T, Sobey CG, Jo DG (2011) Evidence that gamma-secretase-mediated Notch signaling induces neuronal cell death via the nuclear factor-kappaB-Bcl-2-interacting mediator of cell death pathway in ischemic stroke. Mol Pharmacol 80(1):23–31. doi:10.1124/mol.111.071076
Pignataro G, Esposito E, Cuomo O, Sirabella R, Boscia F, Guida N, Di Renzo G, Annunziato L (2011) The NCX3 isoform of the Na+/Ca2+ exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood Flow Metab 31(1):362–370. doi:10.1038/jcbfm.2010.100
Tizon B, Sahoo S, Yu H, Gauthier S, Kumar AR, Mohan P, Figliola M, Pawlik M, Grubb A, Uchiyama Y, Bandyopadhyay U, Cuervo AM, Nixon RA, Levy E (2010) Induction of autophagy by cystatin C: a mechanism that protects murine primary cortical neurons and neuronal cell lines. PLoS ONE 5(3):e9819. doi:10.1371/journal.pone.0009819
Xin H, Li Y, Shen LH, Liu X, Hozeska-Solgot A, Zhang RL, Zhang ZG, Chopp M (2011) Multipotent mesenchymal stromal cells increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes through the sonic hedgehog signaling pathway after stroke (in vitro study). J Cereb Blood Flow Metab 31(11):2181–2188. doi:10.1038/jcbfm.2011.116
Ifediba MA, Medarova Z, Ng SW, Yang J, Moore A (2010) siRNA delivery to CNS cells using a membrane translocation peptide. Bioconjug Chem 21(5):803–806. doi:10.1021/bc900488e
Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J (2011) Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab 31(3):881–893. doi:10.1038/jcbfm.2010.167
Kim HW, Cho KJ, Lee SK, Kim GW (2011) Apoptosis signal-regulating kinase 1 (Ask1) targeted small interfering RNA on ischemic neuronal cell death. Brain Res 1412:73–78. doi:10.1016/j.brainres.2011.07.018
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279(44):45495–45502. doi:10.1074/jbc.M406933200
Halterman MW, Gill M, DeJesus C, Ogihara M, Schor NF, Federoff HJ (2010) The endoplasmic reticulum stress response factor CHOP-10 protects against hypoxia-induced neuronal death. J Biol Chem 285(28):21329–21340. doi:10.1074/jbc.M109.095299
He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH (2012) CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke 43(2):484–490. doi:10.1161/STROKEAHA.111.626432
Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH (2004) Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke 35(10):2412–2417. doi:10.1161/01.STR.0000141162.29864.e9
Bardi G, Tognini P, Ciofani G, Raffa V, Costa M, Pizzorusso T (2009) Pluronic-coated carbon nanotubes do not induce degeneration of cortical neurons in vivo and in vitro. Nanomedicine 5(1):96–104. doi:10.1016/j.nano.2008.06.008
Perez-Carrion MD, Perez-Martinez FC, Merino S, Sanchez-Verdu P, Martinez-Hernandez J, Lujan R, Cena V (2012) Dendrimer-mediated siRNA delivery knocks down Beclin 1 and potentiates NMDA-mediated toxicity in rat cortical neurons. J Neurochem 120(2):259–268. doi:10.1111/j.1471-4159.2011.07556.x
Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg D, Urfer R, Johansson BB, Nikolich K, Wieloch T (2011) The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain 134(Pt 3):732–746. doi:10.1093/brain/awq367
Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R (2011) Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134(Pt 3):704–720. doi:10.1093/brain/awr008
Kitchens CA, McDonald PR, Shun TY, Pollack IF, Lazo JS (2011) Identification of chemosensitivity nodes for vinblastine through small interfering RNA high-throughput screens. J Pharmacol Exp Ther 339(3):851–858. doi:10.1124/jpet.111.184879
Hendruschk S, Wiedemuth R, Aigner A, Topfer K, Cartellieri M, Martin D, Kirsch M, Ikonomidou C, Schackert G, Temme A (2011) RNA interference targeting survivin exerts antitumoral effects in vitro and in established glioma xenografts in vivo. Neuro Oncol 13(10):1074–1089. doi:10.1093/neuonc/nor098
Jin J, Bae KH, Yang H, Lee SJ, Kim H, Kim Y, Joo KM, Seo SW, Park TG, Nam DH (2011) In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem 22(12):2568–2572. doi:10.1021/bc200406n
Mathupala SP (2009) Delivery of small-interfering RNA (siRNA) to the brain. Expert Opin Ther Pat 19(2):137–140. doi:10.1517/13543770802680195
Onishi M, Ichikawa T, Kurozumi K, Date I (2011) Angiogenesis and invasion in glioma. Brain Tumor Pathol 28(1):13–24. doi:10.1007/s10014-010-0007-z
Demuth T, Berens ME (2004) Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 70(2):217–228. doi:10.1007/s11060-004-2751-6
Gagnon KB (2012) High-grade glioma motility reduced by genetic knockdown of KCC3. Cell Physiol Biochem 30(2):466–476. doi:10.1159/000339040
Loftus JC, Ross JT, Paquette KM, Paulino VM, Nasser S, Yang Z, Kloss J, Kim S, Berens ME, Tran NL (2012) miRNA expression profiling in migrating glioblastoma cells: regulation of cell migration and invasion by miR-23b via targeting of Pyk2. PLoS ONE 7(6):e39818. doi:10.1371/journal.pone.0039818
Low J, Blosser W, Dowless M, Ricci-Vitiani L, Pallini R, de Maria R, Stancato L (2012) Knockdown of ubiquitin ligases in glioblastoma cancer stem cells leads to cell death and differentiation. J Biomol Screen 17(2):152–162. doi:10.1177/1087057111422565
Malla RR, Gopinath S, Alapati K, Gorantla B, Gondi CS, Rao JS (2012) uPAR and cathepsin B inhibition enhanced radiation-induced apoptosis in gliomainitiating cells. Neuro Oncol 14(6):745–760. doi:10.1093/neuonc/nos088
Alapati K, Gopinath S, Malla RR, Dasari VR, Rao JS (2012) uPAR and cathepsin B knockdown inhibits radiation-induced PKC integrated integrin signaling to the cytoskeleton of glioma-initiating cells. Int J Oncol 41(2):599–610. doi:10.3892/ijo.2012.1496
Ponnala S, Chetty C, Veeravalli KK, Dinh DH, Klopfenstein JD, Rao JS (2012) Metabolic remodeling precedes mitochondrial outer membrane permeabilization in human glioma xenograft cells. Int J Oncol 40(2):509–518. doi:10.3892/ijo.2011.1255
Zhao Y, Xiao A, diPierro CG, Carpenter JE, Abdel-Fattah R, Redpath GT, Lopes MB, Hussaini IM (2010) An extensive invasive intracranial human glioblastoma xenograft model: role of high level matrix metalloproteinase 9. Am J Pathol 176(6):3032–3049. doi:10.2353/ajpath.2010.090571
Cui NP, Xie SJ, Han JS, Ma ZF, Chen BP, Cai JH (2012) Effective adoptive transfer of haploidentical tumor-specific T cells in B16-melanoma bearing mice. Chin Med J (Engl) 125(5):794–800
Zanotto-Filho A, Braganhol E, Schroder R, de Souza LH, Dalmolin RJ, Pasquali MA, Gelain DP, Battastini AM, Moreira JC (2011) NFkappaB inhibitors induce cell death in glioblastomas. Biochem Pharmacol 81(3):412–424. doi:10.1016/j.bcp.2010.10.014
Hu YY, Zheng MH, Zhang R, Liang YM, Han H (2012) Notch signaling pathway and cancer metastasis. Adv Exp Med Biol 727:186–198. doi:10.1007/978-1-4614-0899-4_14
Raychaudhuri B, Vogelbaum MA (2011) IL-8 is a mediator of NF-kappaB induced invasion by gliomas. J Neurooncol 101(2):227–235. doi:10.1007/s11060-010-0261-2
Smith HW, Marshall CJ (2010) Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11(1):23–36. doi:10.1038/nrm2821
Rao Malla R, Gopinath S, Alapati K, Gorantla B, Gondi CS, Rao JS (2012) Knockdown of cathepsin B and uPAR inhibits CD151 and alpha3beta1 integrin-mediated cell adhesion and invasion in glioma. Mol Carcinog. doi:10.1002/mc.21915
Kim C, Shah BP, Subramaniam P, Lee KB (2011) Synergistic induction of apoptosis in brain cancer cells by targeted codelivery of siRNA and anticancer drugs. Mol Pharm 8(5):1955–1961. doi:10.1021/mp100460h
Niu TK, Cheng Y, Ren X, Yang JM (2010) Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL-induced apoptosis. FEBS Lett 584(16):3519–3524. doi:10.1016/j.febslet.2010.07.018
Zhen HN, Li LW, Zhang W, Fei Z, Shi CH, Yang TT, Bai WT, Zhang X (2007) Short hairpin RNA targeting survivin inhibits growth and angiogenesis of glioma U251 cells. Int J Oncol 31(5):1111–1117
Wang F, Bai HR, Wang J, Bai YZ, Dou CW (2011) Glioma growth inhibition in vitro and in vivo by single chain variable fragments of the transferrin receptor conjugated to survivin small interfering RNA. J Int Med Res 39(5):1701–1712
Jane EP, Premkumar DR, Pollack IF (2011) Bortezomib sensitizes malignant human glioma cells to TRAIL, mediated by inhibition of the NF-{kappa}B signaling pathway. Mol Cancer Ther 10(1):198–208. doi:10.1158/1535-7163.MCT-10-0725
Boado RJ (2005) RNA interference and nonviral targeted gene therapy of experimental brain cancer. NeuroRx 2(1):139–150. doi:10.1602/neurorx.2.1.139
Kunnakkat S, Narayana A (2011) Bevacizumab in the treatment of high-grade gliomas: an overview. Angiogenesis 14(4):423–430. doi:10.1007/s10456-011-9232-2
Chu SH, Feng DF, Zhang H, Chen ET, Duan ZX, Li XY, Li J, Ma YB, Zhu ZA, Qiu JH (2009) c-Met-targeted RNA interference inhibits growth and metastasis of glioma U251 cells in vitro. J Neurooncol 93(2):183–189. doi:10.1007/s11060-008-9772-5
Chen H, Shen X, Guo C, Zhu H, Zhou L, Zhu Y, Wang H, Zheng Y, Huang L (2010) Phosphatase and tensin homolog reconstruction and vascular endothelial growth factor knockdown synergistically inhibit the growth of glioblastoma. Cancer Biother Radiopharm 25(6):713–721. doi:10.1089/cbr.2010.0821
Loew S, Schmidt U, Unterberg A, Halatsch ME (2009) The epidermal growth factor receptor as a therapeutic target in glioblastoma multiforme and other malignant neoplasms. Anticancer Agents Med Chem 9(6):703–715
Michiue H, Eguchi A, Scadeng M, Dowdy SF (2009) Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma. Cancer Biol Ther 8(23):2306–2313
Hsu WM, Che MI, Liao YF, Chang HH, Chen CH, Huang YM, Jeng YM, Huang J, Quon MJ, Lee H, Huang HC, Huang MC (2011) B4GALNT3 expression predicts a favorable prognosis and suppresses cell migration and invasion via beta(1) integrin signaling in neuroblastoma. Am J Pathol 179(3):1394–1404. doi:10.1016/j.ajpath.2011.05.025
Min H, Ghatnekar GS, Ghatnekar AV, You X, Bu M, Guo X, Bu S, Shen B, Huang Q (2012) 2-Methoxyestradiol induced bax phosphorylation and apoptosis in human retinoblastoma cells via p38 MAPK activation. Mol Carcinog 51(7):576–585. doi:10.1002/mc.20825
Mitra M, Kandalam M, Sundaram CS, Verma RS, Maheswari UK, Swaminathan S, Krishnakumar S (2011) Reversal of stathmin-mediated microtubule destabilization sensitizes retinoblastoma cells to a low dose of antimicrotubule agents: a novel synergistic therapeutic intervention. Invest Ophthalmol Vis Sci 52(8):5441–5448. doi:10.1167/iovs.10-6973
Burr DB, Molina SA, Banerjee D, Low DM, Takemoto DJ (2011) Treatment with connexin 46 siRNA suppresses the growth of human Y79 retinoblastoma cell xenografts in vivo. Exp Eye Res 92(4):251–259. doi:10.1016/j.exer.2011.02.003
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012) Age-related macular degeneration. Lancet 379(9827):1728–1738. doi:10.1016/S0140-6736(12)60282-7
Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, Aitchison R, Erlich SS (2012) Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET study). Ophthalmology. doi:10.1016/j.ophtha.2012.03.043
Brafman A, Mett I, Shafir M, Gottlieb H, Damari G, Gozlan-Kelner S, Vishnevskia-Dai V, Skaliter R, Einat P, Faerman A, Feinstein E, Shoshani T (2004) Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci 45(10):3796–3805. doi:10.1167/iovs.04-0052
Ambati J (2011) Age-related macular degeneration and the other double helix. The Cogan lecture. Invest Ophthalmol Vis Sci 52(5):2165–2169. doi:10.1167/iovs.11-7328
Xu B, Descalzi G, Ye HR, Zhuo M, Wang YW (2012) Translational investigation and treatment of neuropathic pain. Mol Pain 8:15. doi:10.1186/1744-8069-8-15
Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A, Ganju P, Wishart W, Hall J (2004) siRNA relieves chronic neuropathic pain. Nucleic Acids Res 32(5):e49. doi:10.1093/nar/gnh044
Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, Lu M, Zhang ZG (2009) Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem 284(34):22680–22689. doi:10.1074/jbc.M109.006551
Dong XW, Goregoaker S, Engler H, Zhou X, Mark L, Crona J, Terry R, Hunter J, Priestley T (2007) Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience 146(2):812–821. doi:10.1016/j.neuroscience.2007.01.054
Cai YQ, Chen SR, Han HD, Sood AK, Lopez-Berestein G, Pan HL (2009) Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J Neurochem 111(4):1000–1010. doi:10.1111/j.1471-4159.2009.06396.x
Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, Kogel B, Rohl T, Schiene K, Schroder W, Seibler J, Kurreck J (2008) Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci 37(3):579–589. doi:10.1016/j.mcn.2007.12.006
Mergler S, Garreis F, Sahlmuller M, Lyras EM, Reinach PS, Dwarakanath A, Paulsen F, Pleyer U (2012) Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochem Cell Biol 137(6):743–761. doi:10.1007/s00418-012-0924-5
Pan Z, Wang Z, Yang H, Zhang F, Reinach PS (2011) TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci 52(1):485–493. doi:10.1167/iovs.10-5801
Xie YT, Du YZ, Yuan H, Hu FQ (2012) Brain-targeting study of stearic acid-grafted chitosan micelle drug-delivery system. Int J Nanomedicine 7:3235–3244. doi:10.2147/IJN.S32701
Lalani J, Rathi M, Lalan M, Misra A (2012) Protein functionalized tramadol-loaded PLGA nanoparticles: preparation, optimization, stability and pharmacodynamic studies. Drug Dev Ind Pharm. doi:10.3109/03639045.2012.684390
Acknowledgments
The T.P. lab is funded by supported by the EU 7th Framework Programme [FP2007-2013] under grant agreements no 223326 and 223524; the EXTRAPLAST IIT project; Epigenomics Flagship Project EPIGEN, MIUR-CNR; PNR-CNR Aging Program 2012-2014; and the CANESTRO project by Regione Toscana. The authors sincerely thank Dr. Paolo Cerioni and Dr. Helen Gallagher and Dr. Darren Finlay for their invaluable help in revising and editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gherardini, L., Bardi, G., Gennaro, M. et al. Novel siRNA delivery strategy: a new “strand” in CNS translational medicine?. Cell. Mol. Life Sci. 71, 1–20 (2014). https://doi.org/10.1007/s00018-013-1310-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1310-8