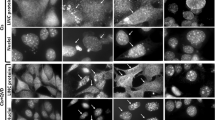
Abstract
Bax and Bak (Bax/Bak) are essential pro-apoptotic proteins of the Bcl-2 family that trigger mitochondrial outer membrane permeabilization (MOMP) in a Bcl-2/Bcl-xL-inhibitable manner. We recently discovered a new stress-related function for Bax/Bak—regulation of nuclear protein redistribution (NPR) from the nucleus to cytoplasm. This effect was independent of Bax/Bak N-terminus exposure and not inhibited by Bcl-xL over-expression. Here, we studied the molecular mechanism governing this novel non-canonical response. Wild-type (WT) and mutant versions of Bax were re-expressed in Bax/Bak double-knockout mouse embryonic fibroblasts and their ability to promote NPR, apoptotic events, and changes in lamin A mobility was examined. Our results show that, in this system, Bax expression was sufficient to restore NPR such as in WT cells undergoing apoptosis. This activity of Bax was uncoupled from cytochrome c release from the mitochondria (indicative of MOMP) and required its membrane localization, α helices 5/6, and the Bcl-2 homology 3 (BH3) domain. Moreover, enrichment of Bax in the nuclear envelope by the so-called Klarsicht/ANC-1/Syne-1 homology domain effectively triggered NPR as in WT Bax, but without inducing MOMP or cell death. Bax-induced NPR was associated with impairment in lamin A mobility, implying a connection between these two nuclear envelope-associated events. Overall, the results indicate a new MOMP-independent, stress-induced Bax function on the nuclear envelope.







Similar content being viewed by others
Abbreviations
- Bax/Bak:
-
Bax and Bak
- DKO:
-
Double-knockout
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FRAP:
-
Fluorescence recovery after photobleaching
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GFP:
-
Green fluorescent protein
- HA:
-
Hemagglutinin
- KASH:
-
Klarsicht/ANC-1/Syne-1 homology
- MEFs:
-
Mouse embryonic fibroblasts
- MOMP:
-
Mitochondrial outer membrane permeabilization
- NE:
-
Nuclear envelope
- NPR:
-
Nuclear protein redistribution
- NPM:
-
Nucleophosmin
- Q-VD-OPH:
-
Quinoline-val-asp(OMe)-CH2-OPH
- RFP:
-
Red fluorescent protein
- WT:
-
Wild-type
References
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59
Wang C, Youle RJ (2009) The role of mitochondria in apoptosis. Annu Rev Genet 43:95–118
Vaux DL (2011) Apoptogenic factors released from mitochondria. Biochim Biophys Acta 1813:546–550
Scorrano L, Korsmeyer SJ (2003) Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun 304:437–444
Lindenboim L, Borner C, Stein R (2011) Nuclear proteins acting on mitochondria. Biochim Biophys Acta 1813:584–596
Lindenboim L, Blacher E, Borner C, Stein R (2010) Regulation of stress-induced nuclear protein redistribution: a new function of Bax and Bak uncoupled from Bcl-x(L). Cell Death Differ 17:346–359
Todaro GJ, Green H (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17:299–313
Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C (2004) Conformational control of Bax localization and apoptotic activity by Pro168. J Cell Biol 164:1021–1032
Lindenboim L, Yuan J, Stein R (2000) Bcl-xS and Bax induce different apoptotic pathways in PC12 cells. Oncogene 19:1783–1793
Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C (2003) Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol 160:53–64
Lindenboim L, Borner C, Stein R (2001) Bcl-x(S) can form homodimers and heterodimers and its apoptotic activity requires localization of Bcl-x(S) to the mitochondria and its BH3 and loop domains. Cell Death Differ 8:933–942
Strasser C, Grote P, Schauble K, Ganz M, Ferrando-May E (2012) Regulation of nuclear envelope permeability in cell death and survival. Nucleus 3:540–551
Ostlund C, Sullivan T, Stewart CL, Worman HJ (2006) Dependence of diffusional mobility of integral inner nuclear membrane proteins on A-type lamins. Biochemistry 45:1374–1382
Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ (2009) Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J cell science 122:4099–4108
Lindenboim L, Kringel S, Braun T, Borner C, Stein R (2005) Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ 12:713–723
Phair RD, Misteli T (2000) High mobility of proteins in the mammalian cell nucleus. Nature 404:604–609
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA et al (1993) Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608
Suzuki M, Youle RJ, Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103:645–654
Zha H, Aime-Sempe C, Sato T, Reed JC (1996) Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem 271:7440–7444
Minn AJ, Kettlun CS, Liang H, Kelekar A, Vander Heiden MG, Chang BS et al (1999) Bcl-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. EMBO J 18:632–643
Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39:615–647
George NM, Evans JJD, Luo X (2007) A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev 21:1937–1948
Heimlich G, McKinnon AD, Bernardo K, Brdiczka D, Reed JC, Kain R, Kronke M, Jurgensmeier JM (2004) Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem J 378:247–255
Upton JP, Valentijn AJ, Zhang L, Gilmore AP (2007) The N-terminal conformation of Bax regulates cell commitment to apoptosis. Cell Death Differ 14:932–942
Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T (1995) Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol CB 5:635–642
Nechushtan A, Smith CL, Hsu YT, Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18:2330–2341
Mitoma J, Ito A (1992) The carboxy-terminal 10 amino acid residues of cytochrome b5 are necessary for its targeting to the endoplasmic reticulum. EMBO J 11:4197–4203
Kalderon D, Roberts BL, Richardson WD, Smith AE (1984) A short amino acid sequence able to specify nuclear location. Cell 39:499–509
Starr DA, Fridolfsson HN (2010) Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 26:421–444
Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP (2002) Nuclear lamins: building blocks of nuclear architecture. Genes Dev 16:533–547
Youle RJ, Karbowski M (2005) Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6:657–663
Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139
Hoetelmans R, van Slooten HJ, Keijzer R, Erkeland S, van de Velde CJ, Dierendonck JH (2000) Bcl-2 and Bax proteins are present in interphase nuclei of mammalian cells. Cell Death Differ 7:384–392
Hoetelmans RW (2004) Nuclear partners of Bcl-2: bax and PML. DNA Cell Biol 23:351–354
Gajkowska B, Motyl T, Olszewska-Badarczuk H, Gniadecki R, Koronkiewicz M (2000) Structural association of Bax with nuclear matrix and cytomatrix revealed by embedment-free immunogold electron microscopy. Cell Biol Int 24:649–656
Gajkowska B, Motyl T, Olszewska-Badarczuk H, Godlewski MM (2001) Expression of BAX in cell nucleus after experimentally induced apoptosis revealed by immunogold and embedment-free electron microscopy. Cell Biol Int 25:725–733
Gajkowska B, Wojewodzka U, Gajda J (2004) Translocation of Bax and Bid to mitochondria, endoplasmic reticulum and nuclear envelope: possible control points in apoptosis. J Mol Histol 35:11–19
Mandal M, Adam L, Mendelsohn J, Kumar R (1998) Nuclear targeting of Bax during apoptosis in human colorectal cancer cells. Oncogene 17:999–1007
Wang X, Olberding KE, White C, Li C (2011) Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ 18:38–47
Gilchrist S, Gilbert N, Perry P, Ostlund C, Worman HJ, Bickmore WA (2004) Altered protein dynamics of disease-associated lamin A mutants. BMC Cell Biol 5:46
Acknowledgments
We thank Prof. Andreas Strasser, The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia, for providing the Bax/Bak DKO MEFs, Prof. Xu Luo, Eppley Institute for Cancer Research, University of Nebraska Medical Center, Omaha, NE, USA, for providing the GFP-Bax 63–65A and GFP-Bax 92–95A plasmids, Prof. Richard J. Youle, Surgical Neurology Branch, NINDS, National Institutes of Health, Bethesda, MD, USA, for providing the Bax S184V plasmid, and Prof. Howard J. Worman, Columbia University, NY, USA, for providing the lamin A-RFP and GFP-mini-nesprin 2G plasmids. This work was supported by the German-Israeli Foundation (to R. Stein and C. Borner), the Spemann Graduate School of Biology and Medicine (SGBM) (GSC-4) and the Excellence Cluster BIOSS (EXC-294) funded by the DFG (to C. Borner).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lindenboim, L., Ferrando-May, E., Borner, C. et al. Non-canonical function of Bax in stress-induced nuclear protein redistribution. Cell. Mol. Life Sci. 70, 3013–3027 (2013). https://doi.org/10.1007/s00018-013-1306-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1306-4