Abstract
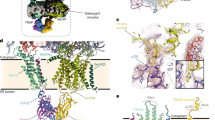
During biosynthesis many membrane and secreted proteins are transported from the endoplasmic reticulum, through the Golgi and on to the plasma membrane in small transport vesicles. These transport vesicles have to undergo budding, movement, tethering, docking, and fusion at each organelle of the biosynthetic pathway. The transport protein particle (TRAPP) complex was initially identified as the tethering factor for endoplasmic reticulum (ER)—derived COPII vesicles, but the functions of TRAPP may extend to other areas of biology. Three forms of TRAPP complexes have been discovered to date, and recent advances in research have provided new insights on the structures and functions of TRAPP. Here we provide a comprehensive review of the recent findings in TRAPP biology.



Similar content being viewed by others
References
Whyte JRC, Munro S (2002) Vesicle tethering complexes in membrane traffic. J Cell Sci 115:2627–2637
Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y et al (2001) TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell 7:433–442
Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D et al (2011) Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473:181–186
Kim Y-G, Raunser S, Munger C, Wagner J, Song Y-L et al (2006) The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell 127:817–830
Sacher M, Yu J, Barrowman J, Scarpa A, Burston J et al (1998) TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J 17:2494–2503
Sacher M, Kim Y-G, Lavie A, Oh B-H, Segev N (2008) The TRAPP complex: Insights into its architecture and function. Traffic 9:2032–2042
Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H et al (2010) Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci 107:7811–7816
Tokarev AA, Taussig D, Sundaram G, Lipatova Z, Liang Y et al (2009) TRAPP II complex assembly requires Trs33 or Trs65. Traffic 10:1831–1844
Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R et al (2006) TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol 8:1263–1269
Cai Y, Chin HF, Lazarova D, Menon S, Fu C et al (2008) The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell 133:1202–1213
Yip CK, Berscheminski J, Walz T (2010) Molecular architecture of the TRAPPII complex and implications for vesicle tethering. Nat Struct Mol Biol 17:1298–1304
Zong M, X-g Wu, Chan CWL, Choi MY, Chan HC et al (2011) The adaptor function of TRAPPC2 in mammalian TRAPPs explains TRAPPC2-associated SEDT and TRAPPC9-associated congenital intellectual disability. PLoS ONE 6:e23350
Liang Y, Morozova N, Tokarev AA, Mulholland JW, Segev N (2007) The role of Trs65 in the Ypt/Rab guanine nucleotide exchange factor function of the TRAPP II complex. Mol Biol Cell 18:2533–2541
Choi C, Davey M, Schluter C, Pandher P, Fang Y et al (2011) Organization and assembly of the TRAPPII complex. Traffic 12:715–725
Scrivens PJ, Shahrzad N, Moores A, Morin A, Brunet S et al (2009) TRAPPC2L is a novel, highly conserved TRAPP-interacting protein. Traffic 10:724–736
Montpetit B, Conibear E (2009) Identification of the novel TRAPP associated protein Tca17. Traffic 10:713–723
Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T et al (2009) mTrs130 Is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI coated vesicles. Mol Biol Cell 20:4205–4215
Sacher M, Barrowman J, Schieltz D, Yates JRI, Ferro-Novick S (2000) Identification and characterization of five new subunits of TRAPP. Eur J Cell Biol 79:71–80
Kummel D, Oeckinghaus A, Wang C, Krappmann D, Heinemann U (2008) Distinct isocomplexes of the TRAPP trafficking factor coexist inside human cells. FEBS Lett 582:3729–3733
Scrivens PJ, Noueihed B, Shahrzad N, Hul S, Brunet S et al (2011) C4orf41 and TTC-15 are mammalian TRAPP components with a role at an early stage in ER-to-Golgi trafficking. Mol Biol Cell 22:2083–2093
Wendler F, Gillingham AK, Sinka R, Rosa-Ferreira C, Gordon DE et al (2010) A genome-wide RNA interference screen identifies two novel components of the metazoan secretory pathway. EMBO J 29:304–314
Behrends C, Sowa ME, Gygi SP, Harper JW (2010) Network organization of the human autophagy system. Nature 466:68–76
Gavin A-C, Bosche M, Krause R, Grandi P, Marzioch M et al (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141–147
Yu S, Satoh A, Pypaert M, Mullen K, Hay JC et al (2006) mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. J Cell Biol 174:359–368
Loh E, Peter F, Subramaniam VN, Hong W (2005) Mammalian Bet3 functions as a cytosolic factor participating in transport from the ER to the Golgi apparatus. J Cell Sci 118:1209–1222
Cai H, Yu S, Menon S, Cai Y, Lazarova D et al (2007) TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature 445:941–944
Bentley M, Liang Y, Mullen K, Xu D, Sztul E et al (2006) SNARE status regulates tether recruitment and function in homotypic COPII vesicle fusion. J Biol Chem 281:38825–38833
Allan B, Moyer B, Balch W (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289:444–448
Price MA (2006) CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev 20:399–410
Cheong JK, Virshup DM (2010) Casein kinase 1: complexity in the family. Int J Biochem Cell Biol 43:465–469
Cronlein J, Reuss S, Vollrath L (1990) Investigations on day-night differences of vesicle densities in synapses of the rat suprachiasmatic nucleus. Neurosci Lett 114:167–172
Mukai S, Matsushima S (1980) Effect of continuous darkness on diurnal rhythms in small vesicles in sympathetic nerve endings of the mouse pineal-quantitative electron microscopic observations. J Neural Transm 47:131–143
Kachi T (1979) Demonstration of circadian rhythm in granular vesicle number in pinealocytes of mice and the effect of light: semi-quantitative electron microscopic study. J Anatomy 129:603–614
Pevet P, Kuyper M (1978) The ultrastructure of pinealocytes in the golden mole (Amblysomus hottentotus) with special reference to the granular vesicles. Cell Tissue Res 191:39–56
Memon A, Hwang S, Deshpande N, Thompson GJ, Herrin D (1995) Novel aspects of the regulation of a cDNA(Arf1) from chlamydomonas with high sequence identity to animal ADP-ribosylation factor 1. Plant Mol Biol 29:567–577
Yu S, Roth MG (2002) Casein kinase I regulates membrane binding by ARF GAP1. Mol Biol Cell 13:2559–2570
Roth MG (1999) Snapshots of ARF1: implications for mechanisms of activation and inactivation. Cell 97:149–152
Siu KY, Yu MK, Wu X, Zong M, Roth MG et al (2011) The non-catalytic carboxyl-terminal domain of ARFGAP1 regulates actin cytoskeleton reorganization by antagonizing the activation of Rac1. PLoS ONE 6:e18458
Wang W, Sacher M, Ferro-Novick S (2000) TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol 151:289–296
Zou S, Liu Y, Zhang X, Chen Y, Ye M, et al (2012) Genetic analysis of exocytic Ypt/Rab GTPases and TRAPP subunits. Genetics (in press)
Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S (2010) TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol 11:759–763
Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE et al (2011) Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci 108:2759–2764
Zhu Y, Hu L, Zhou Y, Yao Q, Liu L et al (2010) Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc Natl Acad Sci 107:4699–4704
Nazarko TY, Huang J, Nicaud J-M, Klionsky DJ, Sibirny AA (2005) Trs85 is required for macroautophagy, pexophagy and cytoplasm to vacuole targeting in Yarrowia lipolytica and Saccharomyces cerevisiae. Autophagy 1:37–45
Meiling-Wesse K, Epple UD, Krick R, Barth H, Appelles A et al (2005) Trs85 (Gsg1), a component of the TRAPP complexes, is required for the organization of the preautophagosomal structure during selective autophagy via the Cvt pathway. J Biol Chem 280:33669–33678
Carlos Martín Zoppino F, Damián Militello R, Slavin I, Álvarez C, Colombo MI (2010) Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic 11:1246–1261
Reggiori F, Wang C-W, Nair U, Shintani T, Abeliovich H et al (2004) Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae. Mol Biol Cell 15:2189–2204
Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y et al (2001) Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell 12:3690–3702
Geng J, Nair U, Yasumura-Yorimitsu K, Klionsky DJ (2010) Post-Golgi sce proteins are required for autophagy in Saccharomyces cerevisiae. Mol Biol Cell 21:2257–2269
Simon G, Prekeris R (2008) Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Transact 36:391–394
Finger FP, White JG (2002) Fusion and fission: membrane trafficking in animal cytokinesis. Cell 108:727–730
Robinett CC, Giansanti MG, Gatti M, Fuller MT (2009) TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J Cell Sci 122:4526–4534
Sciorra VA, Audhya A, Parsons AB, Segev N, Boone C et al (2005) Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol Biol Cell 16:776–793
Qi X, Kaneda M, Chen J, Geitmann A, Zheng H (2011) A specific role for Arabidopsis TRAPPII in post-Golgi trafficking that is crucial for cytokinesis and cell polarity. Plant J 68:234–248
Yoshimura S, Egerer J, Fuchs E, Haas A, Barr F (2007) Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178:363–369
Zong M, Satoh A, Yu MK, Siu KY, Ng WY et al (2012) TRAPPC9 mediates the interaction between p150Glued and COPII vesicles at the target membrane. PLoS ONE 7:e29995
Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ (2005) Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol 7:48–55
Scales SJ, Pepperkok R, Kreis TE (1997) Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90:1137–1148
Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM et al (1997) ER-to-Golgi transport visualized in living cells. Nature 389:81–85
Schroer TA (2004) DYNACTIN. Annu Rev Cell Dev Biol 20:759–779
Watson P, Stephens DJ (2006) Microtubule plus-end loading of p150Glued is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. J Cell Sci 119:2758–2767
Trahey M, Hay JC (2010) Transport vesicle uncoating: it’s later than you think. F1000 Biol Rep 2:47
Tang BL, Peter F, Krijnse-Locker J, Low SH, Griffiths G et al (1997) The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol 17:256–266
Savarirayan R, Thompson E, Gecz J (2003) Spondyloepiphyseal dysplasia tarda. Eur J Hum Genet 11:639–642
Gedeon AK, Colley A, Jamieson R, Thompson EM, Rogers J et al (1999) Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nat Genet 22:400–404
Gecz J, Hillman MA, Gedeon AK, Cox TC, Baker E et al (2000) Gene structure and expression study of the SEDL gene for spondyloepiphyseal dysplasia tarda. Genomics 69:242–251
Sacher M (2003) Membrane traffic fuses with cartilage development. FEBS Lett 550:1–4
Lin Y, Rao S, Yang Y (2008) A novel mutation in the SEDL gene leading to X-linked spondyloepiphyseal dysplasia tarda in a large Chinese pedigree. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 25:150–153
Fiedler J, Merrer ML, Mortier G, Heuertz S, Faivre L et al (2004) X-linked spondyloepiphyseal dysplasia tarda: novel and recurrent mutations in 13 European families. Human Mutat 24:103
Bar-Yosef U, Ohana E, Hershkovitz E, Perlmuter S, Ofir R et al (2004) X-linked spondyloepiphyseal dysplasia tarda: a novel SEDL mutation in a Jewish Ashkenazi family and clinical intervention considerations. Am J Med Genet Part A 125A:45–48
Matsui Y, Yasui N, Ozono K, Yamagata M, Kawabata H et al (2001) Loss of the SEDL gene product (Sedlin) causes X-linked spondyloepiphyseal dysplasia tarda: identification of a molecular defect in a Japanese family. Am J Med Genet 99:328–330
Christie PT, Curley A, Nesbit MA, Chapman C, Genet S et al (2001) Mutational analysis in X-linked spondyloepiphyseal dysplasia tarda. J Clin Endocrinol Metab 86:3233–3236
Choi MY, Chan CCY, Chan D, Luk KDK, Cheah KSE et al (2009) Biochemical consequences of sedlin mutations that cause spondyloepiphyseal dysplasia tarda. Biochem J 423:233–242
Philippe O, Rio M, Carioux A, Plaza J-M, Guigue P et al (2009) Combination of linkage mapping and microarray-expression analysis identifies NF-κB signaling defect as a cause of autosomal-recessive mental retardation. Am J Human Genet 85:903–908
Mochida GH, Mahajnah M, Hill AD, Basel-Vanagaite L, Gleason D et al (2009) A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly. Am J Human Genet 85:897–902
Mir A, Kaufman L, Noor A, Motazacker MM, Jamil T et al (2009) Identification of mutations in TRAPPC9, which encodes the NIK- and IKK-β-binding protein, in nonsyndromic autosomal-recessive mental retardation. Am J Human Genet 85:909–915
Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S (2005) Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J Cell Biol 171:823–833
Hu W-H, Pendergast JS, Mo X-M, Brambilla R, Bracchi-Ricard V et al (2005) NIBP, a novel NIK and IKKβ-binding protein that enhances NF-κB activation. J Biol Chem 280:29233–29241
Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N (2005) A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development 132:3561–3572
Cinaroglu A, Gao C, Imrie D, Sadler KC (2011) Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology 54:495–508
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Gwynn B, Smith R, Rowe L, Taylor B, Peters L (2006) A mouse TRAPP-related protein is involved in pigmentation. Genomics 88:196–203
Kummel D, Walter J, Heck M, Heinemann U, Veit M (2010) Characterization of the self-palmitoylation activity of the transport protein particle component Bet3. Cell Mol Life Sci 67:2653–2664
Kim Y-G, Sohn E, Seo J, Lee K, Lee H et al (2005) Crystal structure of bet3 reveals a novel mechanism for Golgi localization of tethering factor TRAPP. Nat Struct Mol Biol 12:38–45
Chen S, Cai H, Park S-K, Menon S, Jackson CL et al (2011) Trs65p, a subunit of the Ypt1p GEF TRAPPII, interacts with the Arf1p exchange factor Gea2p to facilitate COPI-mediated vesicle traffic. Mol Biol Cell 22:3634–3644
Zhang C, Bowzard J, Greene M, Anido A, Stearns K et al (2002) Genetic interactions link ARF1, YPT31/32 and TRS130. Yeast 19:1075–1086
Yamamoto K, Jigami Y (2002) Mutation of TRS130, which encodes a component of the TRAPP II complex, activates transcription of OCH1 in Saccharomyces cerevisiae. Curr Genet 42:85–93
Ethell IM, Hagihara K, Miura Y, Irie F, Yamaguchi Y (2000) Synbindin, a novel syndecan-2-binding protein in neuronal dendritic spines. J Cell Biol 151:53–68
Zhao S-L, Hong J, Xie Z-Q, Tang J-T, Su W-Y et al (2011) TRAPPC4-ERK2 interaction activates ERK1/2, modulates its nuclear localization and regulates proliferation and apoptosis of colorectal cancer cells. PLoS ONE 6:e23262
Liu X, Wang Y, Zhu H, Zhang Q, Xing X et al (2010) Interaction of Sedlin with PAM14. J Cell Biochem 109:1129–1133
Jeyabalan J, Nesbit MA, Galvanovskis J, Callaghan R, Rorsman P et al (2010) SEDLIN forms homodimers: characterisation of SEDLIN mutations and their interactions with transcription factors MBP1, PITX1 and SF1. PLoS ONE 5:e10646
Fan L, Yu W, Zhu X (2003) Interaction of Sedlin with chloride intracellular channel proteins. FEBS Lett 540:77–80
Acknowledgments
We thank Dr. Michael G. Roth for critical comments of this manuscript. This work is supported by the General Research Fund of the Hong Kong Research Grant Council to S. Y. (grant number 479410).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, S., Liang, Y. A trapper keeper for TRAPP, its structures and functions. Cell. Mol. Life Sci. 69, 3933–3944 (2012). https://doi.org/10.1007/s00018-012-1024-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1024-3