Abstract
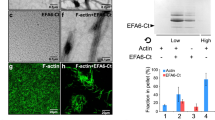
Actin filament-associated protein (AFAP) plays a critical role in the regulation of actin filament integrity, formation and maintenance of the actin network, function of focal contacts, and cell migration. Here, we show that endogenous AFAP was present not only in the cytoskeletal but also in the cytosolic fraction. Depolymerization of actin filaments with cytochalasin D or latrunculin A increased AFAP in the cytosolic fraction. AFAP harbors an actin-binding domain (ABD) in its C-terminus. AFAPΔABD, an AFAP mutant with selective ABD deletion, was mainly in the cytosolic fraction when overexpressed in the cells, which was associated with a disorganized cytoskeleton with reduced stress fibers, accumulation of F-actin on cellular membrane, and formation of actin-rich small dots. Cortactin, a well-known podosome marker, was colocalized with AFAPΔABD in these small dots at the ventral surface of the cell, indicating that these small dots fulfill certain criteria of podosomes. However, these podosome-like small dots did not digest gelatin matrix. This may be due to the reduced interaction between AFAPΔABD and c-Src. When AFAPΔABD-transfected cells were stimulated with phorbol ester, they formed podosome-like structures with larger sizes, less numerous and longer life span, in comparison with wild-type AFAP-transfected cells. These results indicate that the association of AFAP with F-actin through ABD is crucial for AFAP to regulate cytoskeletal structures. The AFAPΔABD, as cytosolic proteins, may be more accessible to the cellular membrane, podosome-like structures, and thus be more interactive for the regulation of cellular functions.










Similar content being viewed by others
References
Baisden JM, Qian Y, Zot HM, Flynn DC (2001) The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene 20:6435–6447
Flynn DC, Leu TH, Reynolds AB, Parsons JT (1993) Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol Cell Biol 13:7892–7900
Lodyga M, Bai XH, Mourgeon E, Han B, Keshavjee S, Liu M (2002) Molecular cloning of actin filament-associated protein: a putative adaptor in stretch-induced Src activation. Am J Physiol Lung Cell Mol Physiol 283:L265–L274
Qian Y, Baisden JM, Westin EH, Guappone AC, Koay TC, Flynn DC (1998) Src can regulate carboxy terminal interactions with AFAP-110, which influence self-association, cell localization and actin filament integrity. Oncogene 16:2185–2195
Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC (2007) AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J Cell Physiol 213:740–749
Qian Y et al (2004) Analysis of the role of the leucine zipper motif in regulating the ability of AFAP-110 to alter actin filament integrity. J Cell Biochem 91:602–620
Baisden JM, Gatesman AS, Cherezova L, Jiang BH, Flynn DC (2001) The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene 20:6607–6616
Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC (2000) The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp Cell Res 255:102–113
Harder J, Xu X, Letourneau P, Lanier LM (2008) The actin cross-linking protein AFAP120 regulates axon elongation in a tyrosine phosphorylation-dependent manner. Neurosci Lett 444:132–136
Walker VG et al (2007) PI3 K activation is required for PMA-directed activation of cSrc by AFAP-110. Am J Physiol Cell Physiol 293:C119–C132
Xu J et al (2007) XB130, a novel adaptor protein for signal transduction. J Biol Chem 282:16401–16412
Buccione R, Orth JD, McNiven MA (2004) Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 5:647–657
Linder S, Aepfelbacher M (2003) Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 13:376–385
Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC (2004) Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol 24:7578–7597
Dorfleutner A, Cho Y, Vincent D, Cunnick J, Lin H, Weed SA, Stehlik C, Flynn DC (2008) Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J Cell Sci 121:2394–2405
Crimaldi L, Courtneidge SA, Gimona M (2009) Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp Cell Res 315:2581–2592
Han B, Bai XH, Lodyga M, Xu J, Yang BB, Keshavjee S, Post M, Liu M (2004) Conversion of mechanical force into biochemical signaling. J Biol Chem 279:54793–54801
Han B, Xiao H, Lodyga M, Bai XH, Jin T, Liu M (2011) Actin filament-associated protein mediated c-Src related SRE/AP-1 transcriptional activation. FEBS Lett 585:471–477
Han B, Lodyga M, Liu M (2005) Ventilator-induced lung injury: role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc 2:181–187
Sawada Y, Sheetz MP (2002) Force transduction by triton cytoskeletons. J Cell Biol 156:609–615
Shiozaki A et al (2011) XB130, a novel adaptor protein, promotes thyroid tumor growth. Am J Pathol 178:391–401
Tamada M, Sheetz MP, Sawada Y (2004) Activation of a signaling cascade by cytoskeleton stretch. Dev Cell 7:709–718
Xiao H, Eves R, Yeh C, Kan W, Xu F, Mak AS, Liu M (2009) Phorbol ester-induced podosomes in normal human bronchial epithelial cells. J Cell Physiol 218:366–375
Xiao H, Bai XH, Kapus A, Lu WY, Mak AS, Liu M (2010) The protein kinase C cascade regulates recruitment of matrix metalloprotease 9 to podosomes and its release and activation. Mol Cell Biol 30:5545–5561
Lodyga M, Bai XH, Kapus A, Liu M (2010) Adaptor protein XB130 is a Rac-controlled component of lamellipodia that regulates cell motility and invasion. J Cell Sci 123:4156–4169
Reznikoff CA, Brankow DW, Heidelberger C (1973) Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33:3231–3238
Tang QQ, Otto TC, Lane MD (2004) Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 101:9607–9611
Qian Y et al (2002) PKC phosphorylation increases the ability of AFAP-110 to cross-link actin filaments. Mol Biol Cell 13:2311–2322
Linder S (2007) The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 17:107–117
West MA, Prescott AR, Chan KM, Zhou Z, Rose-John S, Scheller J, Watts C (2008) TLR ligand-induced podosome disassembly in dendritic cells is ADAM17 dependent. J Cell Biol 182:993–1005
Mukhopadhyay UK, Eves R, Jia L, Mooney P, Mak AS (2009) p53 suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon. Mol Cell Biol 29:3088–3098
Tatin F, Varon C, Genot E, Moreau V (2006) A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci 119:769–781
Chellaiah MA, Kuppuswamy D, Lasky L, Linder S (2007) Phosphorylation of a Wiscott–Aldrich syndrome protein-associated signal complex is critical in osteoclast bone resorption. J Biol Chem 282:10104–10116
Mandal S, Johnson KR, Wheelock MJ (2008) TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp Cell Res 314:3478–3493
Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA (2009) The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell 20:1302–1311
Zhang J et al (2007) AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest 117:2962–2973
Acknowledgments
We thank Dr. Daniel C. Flynn (West Virginia University) for providing AFAP antibody and plasmids. We thank Ms. Serisha Moodley for technical assistance and Ms. Lauren Turrell for proofreading the manuscript. This work was supported by Canadian Institutes of Health Research operating grants MOP-13270 and MOP-42546. HX was supported by Peterborough K. M. Hunter Graduate Studentship for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, H., Han, B., Lodyga, M. et al. The actin-binding domain of actin filament-associated protein (AFAP) is involved in the regulation of cytoskeletal structure. Cell. Mol. Life Sci. 69, 1137–1151 (2012). https://doi.org/10.1007/s00018-011-0812-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0812-5