Abstract
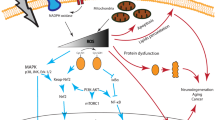
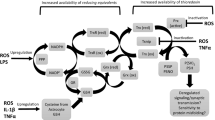
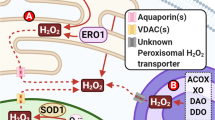
Aberrant or elevated levels of reactive oxygen species (ROS) can mediate deleterious cellular effects, including neuronal toxicity and degeneration observed in the etiology of a number of pathological conditions, including Alzheimer’s and Parkinson’s diseases. Nevertheless, ROS can be generated in a controlled manner and can regulate redox sensitive transcription factors such as NFκB, AP-1 and NFAT. Moreover, ROS can modulate the redox state of tyrosine phosphorylated proteins, thereby having an impact on many transcriptional networks and signaling cascades important for neurogenesis. A large body of literature links the controlled generation of ROS at low-to-moderate levels with the stimulation of differentiation in certain developmental programs such as neurogenesis. In this regard, ROS are involved in governing the acquisition of the neural fate—from neural induction to the elaboration of axons. Here, we summarize and discuss the growing body of literature that describe a role for ROS signaling in neuronal development.

Similar content being viewed by others
References
Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS (2004) Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279(33):34643–34654
Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S (2007) H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem 20(1–4):45–54
Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, Leto TL, Williams MS (2010) The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci Signal 3(133):ra59
Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ (2004) The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24(5):1844–1854
Rhee SG (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312:1882–1883
Burhans WC, Heintz NH (2009) The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med 47(9):1282–1293
Cicchillitti L, Fasanaro P, Biglioli P, Capogrossi MC, Martelli F (2003) Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107, and p130. J Biol Chem 278(21):19509–19517
Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, Quinn MT, Mayaki D, Petrof B, Hussain SN (2008) Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal 10(3):559–574
Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A (2006) NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8(9–10):1473–1484
Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH (2006) Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid Redox Signal 8(9–10):1447–1459
Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR (2006) Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290(4):L661–L673
Kaplan P, Babusikova E, Lehotsky J, Dobrota D (2003) Free radical-induced protein modification and inhibition of Ca2+-ATPase of cardiac sarcoplasmic reticulum. Mol Cell Biochem 248(1–2):41–47
Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P (2006) A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J Biol Chem 281(36):26473–26482
Tirone F, Cox JA (2007) NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett 581(6):1202–1208
Zima AV, Blatter LA (2006) Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71(2):310–321
Gaulden J, Reiter JF (2008) Neur-ons and neur-offs: regulators of neural induction in vertebrate embryos and embryonic stem cells. Hum Mol Genet 17(R1):R60–R66
Fishell G, Mason CA, Hatten ME (1993) Dispersion of neural progenitors within the germinal zones of the forebrain. Nature 362:636–638
Caviness VS Jr, Takahashi T, Nowakowski RS (1995) Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci 18(9):379–383
McConnell SK (1995) Strategies for the generation of neuronal diversity in the developing central nervous system. J Neurosci 15(11):6987–6998
Qian X, Goderie SK, Shen Q, Stern JH, Temple S (1998) Intrinsic programs of patterned cell lineages in isolated vertebrate CNS ventricular zone cells. Development 125(16):3143–3152
Kintner C (2002) Neurogenesis in embryos and in adult neural stem cells. J Neurosci 22(3):639–643
Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH (2000) Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25(2):317–329
Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S (2000) Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28(1):69–80
Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294:1354–1357
Nagler K, Mauch DH, Pfrieger FW (2001) Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol 533:665–679
Kiecker C, Lumsden A (2005) Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci 6(7):553–564
Masland RH (2004) Neuronal cell types. Curr Biol 14(13):R497–R500
Campos-Ortega JA (1993) Mechanisms of early neurogenesis in Drosophila melanogaster. J Neurobiol 24(10):1305–1327
Jan YN, Jan LY (1993) HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell 75(5):827–830
Guillemot F, Joyner AL (1993) Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev 42(3):171–185
Ma Q, Kintner C, Anderson DJ (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87(1):43–52
Johnson JE, Birren SJ, Anderson DJ (1990) Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature 346(6287):858–861
Cabrera CV, Alonso MC (1991) Transcriptional activation by heterodimers of the achaete-scute and daughterless gene products of Drosophila. EMBO J 10(10):2965–2973
Johnson JE, Birren SJ, Saito T, Anderson DJ (1992) DNA binding and transcriptional regulatory activity of mammalian achaete-scute homologous (MASH) proteins revealed by interaction with a muscle-specific enhancer. Proc Natl Acad Sci USA 89(8):3596–3600
Powell LM, Deaton AM, Wear MA, Jarman AP (2008) Specificity of Atonal and Scute bHLH factors: analysis of cognate E box binding sites and the influence of Senseless. Genes Cells 13(9):915–929
Massari ME, Murre C (2000) Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20(2):429–440
Ma Q (2006) Transcriptional regulation of neuronal phenotype in mammals. J Physiol 575:379–387
Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3(7):517–530
Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R (2005) Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res 306(2):343–348
Ross SE, Greenberg ME, Stiles CD (2003) Basic helix-loop-helix factors in cortical development. Neuron 39(1):13–25
Moody SA, Quigg MS, Frankfurter A (1989) Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J Comp Neurol 279(4):567–580
Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J (1999) Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23(2):247–256
Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A (1999) Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci 2(5):461–466
Stavridis MP, Smith AG (2003) Neural differentiation of mouse embryonic stem cells. Biochem Soc Trans 31:45–49
Morrison SJ (2001) Neuronal potential and lineage determination by neural stem cells. Curr Opin Cell Biol 13(6):666–672
Bedard K, Krause HK (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ (2011) Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol 7(2):106–112
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8(9–10):1583–1596
Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME (2006) The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17(9):3978–3988
Buggisch M, Ateghang B, Ruhe C, Strobel C, Lange S, Wartenberg M, Sauer H (2007) Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci 120:885–894
De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F (2000) Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275(30):23227–23233
Grasberger H, Refetoff S (2006) Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281(27):18269–18272
Kennedy KA, Ostrakhovitch EA, Sandiford SD, Dayarathna T, Xie X, Waese EY, Chang WY, Feng Q, Skerjanc IS, Stanford WL, Li SS (2010) Mammalian numb interacting protein1/dual oxidase maturation factor1 directs neuronal fate in stem cells. J Biol Chem 285(23):17974–17985
Kawahara T, Quinn MT, Lambeth JD (2007) Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7:109
Lambeth JD, Kawahara T, Diebold B (2007) Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43(3):319–331
Wang D, De Deken X, Milenkovic M, Song Y, Pirson I, Dumont JE, Miot F (2005) Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J Biol Chem 280(4):3096–3103
Corvilain B, van Sande J, Laurent E, Dumont JE (1991) The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology 128(2):779–785
Takasu N, Yamada T, Shimizu Y, Nagasawa Y, Komiya I (1989) Generation of hydrogen peroxide in cultured porcine thyroid cells: synergistic regulation by cytoplasmic free calcium and protein kinase C. J Endocrinol 120(3):503–508
Raspe E, Laurent E, Corvilain B, Verjans B, Erneux C, Dumont JE (1991) Control of the intracellular Ca(2+)-concentration and the inositol phosphate accumulation in dog thyrocyte primary culture: evidence for different kinetics of Ca(2+)-phosphatidylinositol cascade activation and for involvement in the regulation of H2O2 production. J Cell Physiol 146(2):242–250
Matsubara Y, Ninomiya K, Yasuda Y, Hanawa T, Yagi K, Miyamoto Y, Hatakenaka R, Funatsu T, Ikeda S, Kuwabara M (1986) Clinical evaluation of tumor markers in patients with lung cancer, laying stress on tissue polypeptide antigen (TPA). Nippon Gan Chiryo Gakkai Shi 21(4):744–751
Meier B, Radeke HH, Selle S, Younes M, Sies H, Resch K, Habermehl GG (1989) Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J 263(2):539–545
Smith J, Ladi E, Mayer-Proschel M, Noble M (2000) Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA 97(18):10032–10037
Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA 102(13):4783–4788
Desbordes SC, Placantonakis DG, Ciro A, Socci ND, Lee G, Djaballah H, Studer L (2008) High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell 2(6):602–612
Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H (2011) Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. J Toxicol 2011:405194
Ghosh N, Ghosh R, Mandal SC (2011) Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radic Res 45(8):888–905
Cardaci S, Filomeni G, Rotilio G, Ciriolo MR (2010) p38(MAPK)/p53 signaling axis mediates neuronal apoptosis in response to tetrahydrobiopterin-induced oxidative stress and glucose uptake inhibition: implication for neurodegeneration. Biochem J 430(3):439–451
Nissen C, Ciesielski-Treska J, Hertz L, Mandel P (1973) Regulation of oxygen consumption in neuroblastoma cells: effects of differentiation and of potassium. J Neurochem 20(4):1029–1035
Purves D, McMahan UJ (1972) The distribution of synapses on a physiologically identified motor neuron in the central nervous system of the leech. An electron microscope study after the injection of the fluorescent dye procion yellow. J Cell Biol 55(1):205–220
Kawai S, Yonetani M, Nakamura H, Okada Y (1989) Effects of deprivation of oxygen and glucose on the neural activity and the level of high energy phosphates in the hippocampal slices of immature and adult rat. Brain Res Dev Brain Res 48(1):11–18
Katoh S, Mitsui Y, Kitani K, Suzuki T (1997) Hyperoxia induces the differentiated neuronal phenotype of PC12 cells by producing reactive oxygen species. Biochem Biophys Res Commun 241(2):347–351
Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T (2000) Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem 275(18):13175–13178
Goldsmit Y, Erlich S, Pinkas-Kramarski R (2001) Neuregulin induces sustained reactive oxygen species generation to mediate neuronal differentiation. Cell Mol Neurobiol 21(6):753–769
Tsatmali M, Walcott EC, Crossin KL (2005) Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res 1040(1–2):137–150
Munnamalai V, Suter DM (2009) Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. J Neurochem 108(3):644–661
Qin H, Percival-Smith A, Li C, Jia CY, Gloor G, Li SS (2004) A novel transmembrane protein recruits numb to the plasma membrane during asymmetric cell division. J Biol Chem 279(12):11304–11312
Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, Knaus UG (2009) Heterodimerization controls localization of Duox–DuoxA NADPH oxidases in airway cells. J Cell Sci 122:1238–1247
Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL (2009) Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J 23(4):1205–1218
Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG (2008) Thyroid hormones states and brain development interactions. Int J Dev Neurosci 26(2):147–209
Nitti M, Furfaro AL, Cevasco C, Traverso N, Marinari UM, Pronzato MA, Domenicotti C (2010) PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell Signal 22(5):828–835
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8(1):59–71
Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G (2010) Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol 6(6):411–417
Chan EC, Jiang F, Peshavariya HM, Dusting GJ (2009) Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther 122(2):97–108
Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS (2005) Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. Free Radic Biol Med 38(8):989–1001
Acknowledgements
Work from the authors’ laboratory was supported by grants (to S.S.C.L.) from the Canadian Institute of Health Research and the Canadian Cancer Society. S.C.C.L. holds a Canada Research Chair in Functional Genomics and Cellular Proteomics.
Author information
Authors and Affiliations
Corresponding author
Additional information
K.A.M. Kennedy and S.D.E. Sandiford contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kennedy, K.A.M., Sandiford, S.D.E., Skerjanc, I.S. et al. Reactive oxygen species and the neuronal fate. Cell. Mol. Life Sci. 69, 215–221 (2012). https://doi.org/10.1007/s00018-011-0807-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0807-2