Abstract
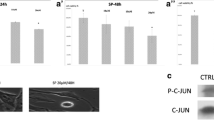
The anti-cancer effect of the c-Jun N-terminal kinase (JNK) inhibitor SP600125 has been well evaluated in human cancer cells. However the role of p21 in SP600125-mediated G2/M distribution is not fully understood. Our results showed that the transcriptional activation of p21 by SP600125 is mediated through the proximal regions of multiple Sp1 sites in the p21 promoter following ERK-dependent phosphorylation of Sp1. In this process, p21 induces endoreduplication through the inhibition of cyclin E/Cdk2 activity at 24 h but does not directly regulate cyclin B1/Cdc2 activity. Furthermore, SP600125 induces the phosphorylation of p21 at Thr 145 through the PI3K/Akt pathway. Akt-mediated phosphorylation of p21 and protection of apoptosis are completely abolished by inhibitors of PI3K and Akt. In summary using time points, we identified the dual functions of p21 as an inhibitor of cell-cycle progression at 24 h and as an anti-apoptotic factor at 48 h.







Similar content being viewed by others
References
Du L, Lyle CS, Obey TB, Gaarde WA, Bennett BL, Chambers TC (2000) Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK activity: evidence that mitotic Bcl-2 phosphorylation is JNK-independent. J Biol Chem 279:11957–11966
Hideshima T, Hayashi T, Chauhan D, Akiyama M, Richardson P, Anderson K (2003) Biologic sequelae of c-Jun NH2-terminal kinase (JNK) activation in multiple myeloma cell lines. Oncogene 22:8797–8801
Jacobs-Helber SM, Sawyer ST (2004) Jun N-terminal kinase promotes proliferation of immature erythroid cells and erythropoietin-dependent cell lines. Blood 104:696–703
Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL (2004) Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene 23:596–604
Wang Q, Wieder R (2004) All-trans retinoic acid potentiates taxotere-induced cell death mediated by Jun-N-terminal kinase in breast cancer cells. Oncogene 23:426–433
Kuntzen C, Sonuc N, De Toni EN, Opelz C, Mucha SR, Gerbes AL, Eichhorst ST (2005) Inhibition of c-Jun-N-terminal-kinase sensitizes tumor cells to CD95-induced apoptosis and induces G2/M cell cycle arrest. Cancer Res 65:6780–6788
Taylor WR, Schonthal AH, Galante J, Stark GR (2001) Regulation of the G2/M transition by p53. J Biol Chem 276:1998–2006
Rozan LM, El-Deiry WS (2007) p53 downstream target genes and tumor suppression: a classical view in evolution. Cell Death Differ 14:3–9
Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9:1149–1163
Waga S, Hannon GJ, Beach D, Stillman B (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574–578
Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, Olson EN, Harper JW, Elledge SJ (1995) p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 267:1024–1027
Esposito F, Cuccovillo F, Vanoni M, Cimino F, Anderson CW, Appella E, Russo T (1997) Redox-mediated regulation of p21waf1/cip1 expression involves a post-transcriptional mechanism and activation of the mitogen-activated protein kinase pathway. Eur J Biochem 245:730–737
Akashi M, Osawa Y, Koeffler HP, Hachiya M (1999) p21WAF1 expression by an activator of protein kinase C is regulated mainly at the post-transcriptional level in cells lacking p53: important role of RNA stabilization. Biochem J 337:607–616
Gartel AL, Tyner AL (1999) Transcriptional regulation of the p21(WAF1/CIP1) gene. Exp Cell Res 246:280–289
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
Rössig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S (2001) Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol 21:5644–5657
Li Y, Dowbenko D, Lasky LA (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277:11352–11361
Hwang-Verslues WW, Sladek FM (2008) Nuclear receptor hepatocyte nuclear factor 4alpha1 competes with oncoprotein c-Myc for control of the p21/WAF1 promoter. Mol Endocrinol 22:78–90
Datto MB, Yu Y, Wang XF (1995) Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J Biol Chem 270:28623–28628
Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ (1998) Increasing complexity of Ras signaling. Oncogene 17:1395–1413
Moreno S, Hayles J, Nurse P (1989) Regulation of p34cdc2 protein kinase during mitosis. Cell 58:361–372
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3:245–252
Leslie NR, Downes CP (2004) PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382:1–11
Tsurimoto T (1998) PCNA, a multifunctional ring on DNA. Biochim Biophys Acta 1443:23–39
Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S (1999) Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J 18:1223–1234
Coqueret O (2003) New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13:65–70
Harms C, Albrecht K, Harms U, Seidel K, Hauck L, Baldinger T, Hübner D, Kronenberg G, An J, Ruscher K, Meisel A, Dirnagl U, von Harsdorf R, Endres M, Hörtnagl H (2007) Phosphatidylinositol 3-Akt-kinase-dependent phosphorylation of p21Waf1/Cip1 as a novel mechanism of neuroprotection by glucocorticoids. J Neurosci 27:4562–4571
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252
Lin A (2003) Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 25:17–24
Xiong Y, Zhang H, Beach D (1993) Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev 7:1572–1583
Aikin R, Maysinger D, Rosenberg L (2004) Cross-talk phosphatidylinositol 3-kinase/AKT and c-Jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology 145:4522–4531
Allen RT, Krueger KD, Dhume A, Agrawal DK (2005) Sustained Akt/PKB activation and transient attenuation of c-jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis 10:525–535
Fornoni A, Pileggi A, Molano RD, Sanabria NY, Tejada T, Gonzalez-Quintana J, Ichii H, Inverardi L, Ricordi C, Pastori RL (2008) Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia 51:298–308
Moon DO, Kim MO, Choi YH, Kim ND, Chang JH, Kim GY (2008) Bcl-2 overexpression attenuates SP600125-induced apoptosis in human leukemia U937 cells. Cancer Lett 264:316–325
Suzuki A, Tsutomi Y, Yamamoto N, Shibutani T, Akahane K (1999) Mitochondrial regulation of cell death: mitochondria are essential for procaspase 3-p21 complex formation to resist fas-mediated cell death. Mol Cell Biol 19:3842–3847
Xu SQ, El-Deiry WS (2000) p21WAF1/CIP1 inhibits initiator caspase cleavage by TRAIL death receptor DR4. Biochem Biophys Res Commun 269:179–190
Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S (1999) Apoptosis inhibitory activity of cytoplasmic p21 (Cip1/WAF1) in monocytic differentiation. EMBO J 18:1223–1234
Liang J, Slingerland JM (2003) Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2:339–345
Maddika S, Ande SR, Wiechec E, Hansen LL, Wesselborg S, Los M (2008) Akt-mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J Cell Sci 121:979–988
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0016098). We greatly appreciate the gift of HCT116 p21+/+ and HCT116 p21−/− cells from Prof. Deung Y. Shin (Dankook University College of Medicine, Chungnam, Republic of Korea).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Moon, DO., Choi, Y.H. & Kim, GY. Role of p21 in SP600125-induced cell cycle arrest, endoreduplication, and apoptosis. Cell. Mol. Life Sci. 68, 3249–3260 (2011). https://doi.org/10.1007/s00018-011-0626-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0626-5