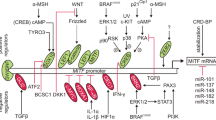
Abstract
Since bone morphogenetic proteins (BMPs) play an important role in melanoma progression, we aimed to determine the molecular mechanisms leading to overexpression of BMP4 in melanoma cells compared to normal melanocytes. With our experimental approach we revealed that loss of expression of a microRNA represents the starting point for a signaling cascade finally resulting in overexpression of BMP4 in melanoma cells. In detail, strongly reduced expression of the microRNA miR-196a in melanoma cells compared to healthy melanocytes leads to enhanced HOX-B7 mRNA and protein levels, which subsequently raise Ets-1 activity by inducing basic fibroblast growth factor (bFGF). Ets-1 finally accounts for induction of BMP4 expression. We were furthermore able to demonstrate that bFGF-mediated induction of migration is achieved via activation of BMP4, thus determining BMP4 as major modulator of migration in melanoma. In summary, our study provides insights into the early steps of melanoma progression and might thereby harbor therapeutic relevance.






Similar content being viewed by others
References
Mehler MF, Mabie PC, Zhang D, Kessler JA (1997) Bone morphogenetic proteins in the nervous system. Trends Neurosci 20:309–317
Turgeman G, Pittman DD, Muller R, Kurkalli BG, Zhou S, Pelled G, Peyser A, Zilberman Y, Moutsatsos IK, Gazit D (2001) Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med 3:240–251
Hogan BL (1996) Bone morphogenetic proteins in development. Curr Opin Genet Dev 6:432–438
Balemans W, Van Hul W (2002) Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250:231–250
Yamamoto Y, Oelgeschlager M (2004) Regulation of bone morphogenetic proteins in early embryonic development. Naturwissenschaften 91:519–534
Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, Korc M (1999) Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology 116:1202–1216
Kiyozuka Y, Nakagawa H, Senzaki H, Uemura Y, Adachi S, Teramoto Y, Matsuyama T, Bessho K, Tsubura A (2001) Bone morphogenetic protein-2 and type IV collagen expression in psammoma body forming ovarian cancer. Anticancer Res 21:1723–1730
Ye L, Lewis-Russell JM, Kyanaston HG, Jiang WG (2007) Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol 22:1129–1147
Fong YC, Li TM, Wu CM, Hsu SF, Kao ST, Chen RJ, Lin CC, Liu SC, Wu CL, Tang CH (2008) BMP-2 increases migration of human chondrosarcoma cells via PI3K/Akt pathway. J Cell Physiol 217:846–855
Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR (2008) Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer 8:806–812
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27:6322–6333
Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK (2005) Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res 65:448–456
Hsu MY, Rovinsky S, Penmatcha S, Herlyn M, Muirhead D (2005) Bone morphogenetic proteins in melanoma: angel or devil? Cancer Metastasis Rev 24:251–263
Rothhammer T, Braig S, Bosserhoff AK (2008) Bone morphogenetic proteins induce expression of metalloproteinases in melanoma cells and fibroblasts. Eur J Cancer 44:2526–2534
Rothhammer T, Wild PJ, Meyer S, Bataille F, Pauer A, Klinkhammer-Schalke M, Hein R, Hofstaedter F, Bosserhoff AK (2007) Bone morphogenetic protein 7 (BMP7) expression is a potential novel prognostic marker for recurrence in patients with primary melanoma. Cancer Biomark 3:111–117
Boswell BA, Lein PJ, Musil LS (2008) Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell 19:2631–2641
Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S (2005) Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone 36:399–407
Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, McGuire J (1988) Paracrine stimulation of melanocytes by keratinocytes through basic fibroblast growth factor. Ann N Y Acad Sci 548:180–190
Giehl KA, Nagele U, Volkenandt M, Berking C (2007) Protein expression of melanocyte growth factors (bFGF, SCF) and their receptors (FGFR-1, c-kit) in nevi and melanoma. J Cutan Pathol 34:7–14
Halaban R, Kwon BS, Ghosh S, Delli Bovi P, Baird A (1988) bFGF as an autocrine growth factor for human melanomas. Oncogene Res 3:177–186
Scott G, Stoler M, Sarkar S, Halaban R (1991) Localization of basic fibroblast growth factor mRNA in melanocytic lesions by in situ hybridization. J Invest Dermatol 96:318–322
Wang Y, Becker D (1997) Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 3:887–893
Meier F, Caroli U, Satyamoorthy K, Schittek B, Bauer J, Berking C, Moller H, Maczey E, Rassner G, Herlyn M, Garbe C (2003) Fibroblast growth factor-2 but not Mel-CAM and/or beta3 integrin promotes progression of melanocytes to melanoma. Exp Dermatol 12:296–306
Care A, Silvani A, Meccia E, Mattia G, Stoppacciaro A, Parmiani G, Peschle C, Colombo MP (1996) HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol 16:4842–4851
Deschamps J, Meijlink F (1992) Mammalian homeobox genes in normal development and neoplasia. Crit Rev Oncog 3:117–173
Abe M, Hamada J, Takahashi O, Takahashi Y, Tada M, Miyamoto M, Morikawa T, Kondo S, Moriuchi T (2006) Disordered expression of HOX genes in human non-small cell lung cancer. Oncol Rep 15:797–802
Lopez R, Garrido E, Pina P, Hidalgo A, Lazos M, Ochoa R, Salcedo M (2006) HOXB homeobox gene expression in cervical carcinoma. Int J Gynecol Cancer 16:329–335
Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, Volinia S, Liu CG, Schnittger S, Haferlach T, Liso A, Diverio D, Mancini M, Meloni G, Foa R, Martelli MF, Mecucci C, Croce CM, Falini B (2008) Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA 105:3945–3950
Chopra VS, Mishra RK (2006) “Mir”acles in hox gene regulation. Bioessays 28:445–448
Mansfield JH, Harfe BD, Nissen R, Obenauer J, Srineel J, Chaudhuri A, Farzan-Kashani R, Zuker M, Pasquinelli AE, Ruvkun G, Sharp PA, Tabin CJ, McManus MT (2004) MicroRNA-responsive ‘sensor’ transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat Genet 36:1079–1083
Yekta S, Shih IH, Bartel DP (2004) MicroRNA-directed cleavage of HOXB8 mRNA. Science 304:594–596
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Nielsen CB, Shomron N, Sandberg R, Hornstein E, Kitzman J, Burge CB (2007) Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. RNA 13:1894–1910
Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E (2006) mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 20:1885–1898
Wu L, Fan J, Belasco JG (2006) MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA 103:4034–4039
Pillai RS, Bhattacharyya SN, Filipowicz W (2007) Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 17:118–126
Standart N, Jackson RJ (2007) MicroRNAs repress translation of m7Gppp-capped target mRNAs in vitro by inhibiting initiation and promoting deadenylation. Genes Dev 21:1975–1982
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103:2257–2261
Schickel R, Boyerinas B, Park SM, Peter ME (2008) MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 27:5959–5974
Mueller DW, Rehli M, Bosserhoff AK (2009) miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol 129:1740–1751
Muller DW, Bosserhoff AK (2008) Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene 27:6698–6706
Mueller DW, Bosserhoff AK (2009) Role of miRNAs in the progression of malignant melanoma. Br J Cancer 101:551–556
Jacob K, Wach F, Holzapfel U, Hein R, Lengyel E, Buettner R, Bosserhoff AK (1998) In vitro modulation of human melanoma cell invasion and proliferation by all-trans-retinoic acid. Melanoma Res 8:211–219
Rothhammer T, Hahne JC, Florin A, Poser I, Soncin F, Wernert N, Bosserhoff AK (2004) The Ets-1 transcription factor is involved in the development and invasion of malignant melanoma. Cell Mol Life Sci 61:118–128
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Madry H, Emkey G, Zurakowski D, Trippel SB (2004) Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J Gene Med 6:238–245
Groitl P, Dobner T (2007) Construction of adenovirus type 5 early region 1 and 4 virus mutants. Methods Mol Med 130:29–39
Arndt S, Poser I, Moser M, Bosserhoff AK (2007) Fussel-15, a novel Ski/Sno homolog protein, antagonizes BMP signaling. Mol Cell Neurosci 34:603–611
Montesano R, Sarkozi R, Schramek H (2008) Bone morphogenetic protein-4 strongly potentiates growth factor-induced proliferation of mammary epithelial cells. Biochem Biophys Res Commun 374:164–168
Katoh M (2007) Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev 3:30–38
Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Donatien P, Crombleholme TM, Herlyn M (2000) Human melanoma progression in skin reconstructs: biological significance of bFGF. Am J Pathol 156:193–200
Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE (1995) Growth control of melanoma cells and melanocytes by cytokines. Recent Results Cancer Res 139:169–182
Griffiths-Jones S (2006) miRBase: the microRNA sequence database. Methods Mol Biol 342:129–138
Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB (2003) Prediction of mammalian microRNA targets. Cell 115:787–798
Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N (2005) Combinatorial microRNA target predictions. Nat Genet 37:495–500
Miyazono K, Miyazawa K (2002) Id: a target of BMP signaling. Sci STKE, pe40
Iwasaka C, Tanaka K, Abe M, Sato Y (1996) Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol 169:522–531
Becker D, Meier CB, Herlyn M (1989) Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J 8:3685–3691
Tsunoda S, Nakamura T, Sakurai H, Saiki I (2007) Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci 98:541–548
Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J (2003) The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis 24:1445–1454
Yamashita T, Tazawa S, Yawei Z, Katayama H, Kato Y, Nishiwaki K, Yokohama Y, Ishikawa M (2006) Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol 28:931–938
Wu X, Chen H, Parker B, Rubin E, Zhu T, Lee JS, Argani P, Sukumar S (2006) HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res 66:9527–9534
Care A, Silvani A, Meccia E, Mattia G, Peschle C, Colombo MP (1998) Transduction of the SkBr3 breast carcinoma cell line with the HOXB7 gene induces bFGF expression, increases cell proliferation and reduces growth factor dependence. Oncogene 16:3285–3289
Acknowledgments
This work was supported by the Deutsche Krebshilfe e.V. (grant number 108777) and the Deutsche Forschungsgemeinschaft (grant number BO1573/16-1). We thank Prof. Soncin (Lille, France) for providing the Ets-1 construct, Dr. Madry (Homburg, Germany) for providing the bFGF expression plasmid; Dr. Moser (Martinsried, Germany) for providing the BMP4 promoter reporter constructs; and Sibylla Lodermeyer for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Braig and D. W. Mueller contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2010_394_MOESM1_ESM.jpeg
Fig. S1 Confirmation of bFGF overexpression in Mel Im melanoma cells transduced with a bFGF expressing adenovirus by qRT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 229 kb)
18_2010_394_MOESM2_ESM.jpeg
Fig. S2 Compilation of photographs exemplary for filter areas counted in the Boyden chamber assays described in Fig. 1C (JPEG 2028 kb)
18_2010_394_MOESM3_ESM.tif
Fig. S3 Enhanced migratory potential of NHEMs in Boyden chamber assays after treatment with recombinant BMP4 protein (100ng/ml) for 24h. Untreated NHEMs served as a control. Bars show the means ± SD of one out of three independent experiments, measurements were performed in triplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (TIFF 10503 kb)
18_2010_394_MOESM4_ESM.jpeg
Fig. S4 (A) bFGF and (B) BMP4 protein expression in two cell lines derived from primary melanoma lesions and two cell lines derived from metastatic melanomas, respectively. Absolute protein levels were measured by ELISA. Protein levels of bFGF and BMP4 in standard DMEM medium were determined and subsequently subtracted from total values. After subtraction of bFGF and BMP4 levels measured in the melanocyte growth medium used, NHEMs showed no endogenous expression of bFGF and BMP4, respectively. One representative experiment is shown out of three replicates (JPEG 1159 kb)
18_2010_394_MOESM5_ESM.jpeg
Fig. S5 HOX-B7 mRNA expression is reduced in the melanoma cell line Mel Im after transfection of either siHOX-B7#1 or siHOX-B7#4 compared to control transfected cells as shown by quantitative RT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 629 kb)
18_2010_394_MOESM6_ESM.jpeg
Fig. S6 Absolute bFGF and BMP4 protein levels of Mel Im cells transfected with siRNA against HOX-B7 for 48h. One representative out of three independent experiments is shown (JPEG 615 kb)
18_2010_394_MOESM7_ESM.jpeg
Fig. S7 The miR-196a re-expressing cell clones exhibited decreased Id1 mRNA expression compared to pcDNA3 empty vector control cells as shown by quantitative RT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 632 kb)
Rights and permissions
About this article
Cite this article
Braig, S., Mueller, D.W., Rothhammer, T. et al. MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell. Mol. Life Sci. 67, 3535–3548 (2010). https://doi.org/10.1007/s00018-010-0394-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0394-7