Abstract
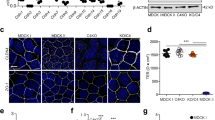
Tight junctions control paracellular permeability. Here, we analyzed the impact of residues in the second extracellular loop (ECL2) of mouse claudin-5 on paracellular permeability. Stable expression of claudin-5wild type in MDCK-II cells—but not that of mutants R145A, Y148A, Y158A or E159Q—increased transepithelial electrical resistance and decreased fluorescein permeation. Expression of claudin-5Y148A, Y158A or E159Q enhanced permeability of FITC-dextran10 kDa, which was unchanged in cells expressing claudin-5wild type or claudin-5R145A. In contrast, targeting to tight junctions, strand morphology and tight junction assembly were unchanged. It is concluded that R145 is unessential for trans-interaction of claudin-5, but necessary for tightening against small solutes and ions. The highly conserved residues Y148, Y158 and E159 in ECL2 of claudin-5 contribute to homo- and/or heterophilic trans-interaction between classic claudins and thereby tighten the paracellular space against ions, small and large molecules. These results provide novel insights into the molecular function of tight junctions.






Similar content being viewed by others
Abbreviations
- Cld:
-
Claudin
- Da:
-
Dalton
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ECL:
-
Extracellular loop
- FITC:
-
Fluorescein isothiocyanate
- HBSS:
-
Hank’s buffered salt solution
- HRP:
-
Horseradish peroxidase
- MDCK:
-
Madin-Darby canine kidney
- PBS:
-
Phosphate buffered saline
- Pfluorescein :
-
Permeability coefficient of fluorescein
- PFD10 :
-
Permeability coefficient of 10 kDa FITC-dextran
- SEM:
-
Standard error of the mean
- TEER:
-
Transepithelial electrical resistance
- TJ:
-
Tight junctions
References
Farquhar MG, Palade GE (1963) Junctional complexes in various epithelia. J Cell Biol 17:375–412
Staehelin LA (1973) Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci 13:763–786
Furuse M, Sasaki H, Fujimoto K, Tsukita S (1998) A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143:391–401
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim Biophys Acta 1778:631–645
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660
Furuse M, Sasaki H, Tsukita S (1999) Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147:891–903
Van Itallie CM, Anderson JM (2006) Claudins and epithelial paracellular transport. Annu Rev Physiol 68:403–429
Van Itallie CM, Fanning AS, Anderson JM (2003) Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285:F1078–F1084
Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD (2009) Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 133:111–127
Wen H, Watry DD, Marcondes MC, Fox HS (2004) Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol 24:8408–8417
Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE (2008) Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 22:146–158
Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J (2006) On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci 63:505–514
Andreeva AY, Krause E, Muller EC, Blasig IE, Utepbergenov DI (2001) Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem 276:38480–38486
Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, de Boer AG, Breimer DD (2001) Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci 12:215–222
Krug SM, Fromm M, Gunzel D (2009) Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys J 97:2202–2211
Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 20:3713–3724
Konishi Y, Hagiwara K, Shimizu M (2002) Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci Biotechnol Biochem 66:2449–2457
Mack AF, Wolburg H (2006) Growing axons in fish optic nerve are accompanied by astrocytes interconnected by tight junctions. Brain Res 1103:25–31
Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter AH, Schulzke JD, Fromm M (2005) Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res 321:89–96
Hou J, Gomes AS, Paul DL, Goodenough DA (2006) Study of claudin function by RNA interference. J Biol Chem 281:36117–36123
Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM (2002) Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283:C142–C147
Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH (2007) The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun 357:87–91
Hou J, Paul DL, Goodenough DA (2005) Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118:5109–5118
Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E (2007) Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol 170:1389–1397
Alexandre MD, Lu Q, Chen YH (2005) Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci 118:2683–2693
Angelow S, Schneeberger EE, Yu AS (2007) Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 215:147–159
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147:1351–1363
Angelow S, Ahlstrom R, Yu AS (2008) Biology of Claudins. Am J Physiol Renal Physiol 295:F867–F876
Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG (2003) Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285:L1166–L1178
Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M (2007) Regulation of heterotypic claudin compatibility. J Biol Chem 282:30005–30013
Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA (2008) Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118:619–628
Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA (2009) Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA 106:15350–15355
Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976
Furuse M, Furuse K, Sasaki H, Tsukita S (2001) Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153:263–272
Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S (2010) Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci USA 107:1425–1430
Winkler L, Gehring C, Wenzel A, Müller SL, Piehl C, Krause G, Blasig IE, Piontek J (2009) Molecular determinants of the interaction between Clostridium perfringens enterotoxin fragments and claudin-3. J Biol Chem 284:18863–18872
Acknowledgments
We thank Barbara Eilemann for technical assistance. We gratefully acknowledge the help of Ria Knittel in freeze-fracturing. This work was funded by DFG BL308/7-3, 7-4 and PI 837/2-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Piehl and J. Piontek contributed equally to this work.
Electronic supplementary material
Fig. S1.
The amino acid substitution Y148A in claudin-5 does not affect disassembly nor reassembly of tight junctions. (A) MDCK-II cells, stably transfected with Cld5wt or Cld5Y148A were cultured until confluent before standard medium was replaced by medium containing 2mM EGTA for Ca2+-delpetion. The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and the nuclei (blue) were detected before, 0.5, 1, 2 and 3 h after removal of Ca2+ by immunostaining and subsequent confocal microscopy. With increasing time of Ca2+-depletion the detected amount of Cld5 and Cld1 in the plasma membrane decreased and that in intracellular compartments increased for Cld5wt- as well as for Cld5Y148A-expressing cells. At no time point internalization of Cld5 or Cld1 was more pronounced for Cld5Y148A- than for Cld5wt-expressing cells (B) The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and nuclei (blue) were detected at 0, 0.25, 0.5, 1, 2, 3 and 4 h after media exchange to Ca2+ containing standard medium. Between Cld5wt- and Cld5Y148A-expressing cells, no difference in the reappearance at the plasma membrane was detectable for either Cld1 or Cld5. (JPEG 3.61 MB)
Fig. S1.
The amino acid substitution Y148A in claudin-5 does not affect disassembly nor reassembly of tight junctions. (A) MDCK-II cells, stably transfected with Cld5wt or Cld5Y148A were cultured until confluent before standard medium was replaced by medium containing 2mM EGTA for Ca2+-delpetion. The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and the nuclei (blue) were detected before, 0.5, 1, 2 and 3 h after removal of Ca2+ by immunostaining and subsequent confocal microscopy. With increasing time of Ca2+-depletion the detected amount of Cld5 and Cld1 in the plasma membrane decreased and that in intracellular compartments increased for Cld5wt- as well as for Cld5Y148A-expressing cells. At no time point internalization of Cld5 or Cld1 was more pronounced for Cld5Y148A- than for Cld5wt-expressing cells (B) The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and nuclei (blue) were detected at 0, 0.25, 0.5, 1, 2, 3 and 4 h after media exchange to Ca2+ containing standard medium. Between Cld5wt- and Cld5Y148A-expressing cells, no difference in the reappearance at the plasma membrane was detectable for either Cld1 or Cld5. (JPEG 4.70 MB)
Rights and permissions
About this article
Cite this article
Piehl, C., Piontek, J., Cording, J. et al. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell. Mol. Life Sci. 67, 2131–2140 (2010). https://doi.org/10.1007/s00018-010-0332-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0332-8