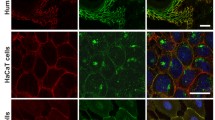
Abstract
We provide the first description of the desmosome network in keratinocytes using a systems level approach. The desmo-adhesome consists of 59 proteins connected by 128 direct interactions and forms different functional subnets. Whilst the structure appears to be extremely robust against random perturbations, network fragmentation analysis suggests that the desmo-adhesome is susceptible to targeted attacks. To confirm this prediction, we applied this model to the autoimmune disease Pemphigus Vulgaris (PV), a paradigm of external perturbation of the desmosome. Our analysis showed that the adaptor protein plakophilin (Pkp) 3 was in the highest percentile group for both connectivity rate and gene expression changes in experimental PV. This observation led us to speculate that Pkp3 was crucial in desmosomal remodelling, and therefore we designed the experiments to verify this hypothesis. Our data demonstrate that, whilst Pkp3 is important in conferring adhesive strength to keratinocytes, it also acts as a central molecule mediating cell–cell detachment induced by PV IgG.









Similar content being viewed by others
References
Bruggeman FJ, Westerhoff HV (2007) The nature of systems biology. Trends Microbiol 15:45–50
Hess EL (1970) Origins of molecular biology. Science 168:664–669
Almaas E (2007) Biological impacts and context of network theory. J Exp Biol 210:1548–1558
Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33:49–54
Ramaswamy S (2007) Rational design of cancer-drug combinations. N Engl J Med 357:299–300
Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 100:2610–2615
Nelson WJ (2008) Regulation of cell–cell adhesion by the cadherin–catenin complex. Biochem Soc Trans 36:149–155
Angst BD, Marcozzi C, Magee AI (2001) The cadherin superfamily: diversity in form and function. J Cell Sci 114:629–641
Cirillo N (2009) Pathophysiology of the desmosome. Research Signpost, Kerala, India
Garrod D, Chidgey M (2008) Desmosome structure, composition and function. Biochim Biophys Acta 1778:572–587
Miera A, Foshay K, Stewart A, Gallicano GI (2009) Development of a sticky situation: the role of the desmosome in epidermal morphogenesis. In: Cirillo N (ed) Pathophysiology of the desmosome. Research Signpost, Kerala, pp 33–61
Payne AS, Hanakawa Y, Amagai M, Stanley JR (2004) Desmosomes and disease: pemphigus and bullous impetigo. Curr Opin Cell Biol 16:536–543
Green KJ, Simpson CL (2007) Desmosomes: new perspectives on a classic. J Invest Dermatol 127:2499–2515
Lanza A, Cirillo N, Femiano F, Gombos F (2006) How does acantholysis occur in pemphigus vulgaris: a critical review. J Cutan Pathol 33:401–412
Waschke J (2008) The desmosome and pemphigus. Histochem Cell Biol 130:21–54
Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9:858–867
Paris L, Bazzoni G (2008) The protein interaction network of the epithelial junctional complex: a system-level analysis. Mol Biol Cell 19:5409–5421
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Pieroni E, de la Fuente van Bentem S, Mancosu G, Capobianco E, Hirt H, de la Fuente A (2008) Protein networking: insights into global functional organization of proteomes. Proteomics 8:799–816
Albert R, Jeong H, Barabasi AL (2000) Error and attack tolerance of complex networks. Nature 406:378–382
Lanza A, Cirillo N, Rossiello R, Rienzo M, Cutillo L, Casamassimi A, de Nigris F, Schiano C, Rossiello L, Femiano F, Gombos F, Napoli C (2008) Evidence of key role of Cdk2 overexpression in pemphigus vulgaris. J Biol Chem 283:8736–8745
Cirillo N, Lanza M, De Rosa A, Femiano F, Gombos F, Lanza A (2008) At least three phosphorylation events induced by pemphigus vulgaris sera are pathogenically involved in keratinocyte acantholysis. Int J Immunopathol Pharmacol 21:189–195
Cirillo N, Gombos F, Lanza A (2007) Pemphigus vulgaris immunoglobulin G can recognize a 130 000 MW antigen other than desmoglein 3 on peripheral blood mononuclear cell surface. Immunology 121:377–382
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771
Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Dotto GP (1998) Tyrosine phosphorylation and Src-family kinases control keratinocyte cell–cell adhesion. J Cell Biol 141:1449–1465
Cirillo N, Lanza M, Femiano F, Gaeta GM, De Rosa A, Gombos F, Lanza A (2007) If pemphigus vulgaris IgG are the cause of acantholysis, new IgG-independent mechanisms are the concause. J Cell Physiol 212:563–567
Cirillo N, Lanza M, De Rosa A, Cammarota M, La Gatta A, Gombos F, Lanza A (2008) The most widespread desmosomal cadherin, desmoglein 2, is a novel target of caspase 3-mediated apoptotic machinery. J Cell Biochem 103:598–606
Lanza A, Santoro R, De Rosa A, Gaeta GM, Gombos F, Cirillo N (2007) Inhibition of protein phosphorylation, but not synthesis nor lysosomal degradation, prevents keratinocyte adhesion loss induced by pemphigus vulgaris serum. J Stomatol Invest 1:25–32
Bragulla HH, Homberger DG (2009) Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 214:516–559
Hesse M, Watson ED, Schwaluk T, Magin TM (2005) Rescue of keratin 18/19 doubly deficient mice using aggregation with tetraploid embryos. Eur J Cell Biol 84:355–361
Waschke J (2008) Direct interference with desmoglein binding in pemphigus. J Stomatol Invest 2:53–55
Sklyarova T, Bonné S, D’Hooge P, Denecker G, Goossens S, De Rycke R, Borgonie G, Bösl M, van Roy F, van Hengel J (2008) Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J Invest Dermatol 128:1375–1385
Nguyen VT, Arredondo J, Chernyavsky AI, Kitajima Y, Pittelkow M, Grando SA (2004) Pemphigus vulgaris IgG and methylprednisolone exhibit reciprocal effects on keratinocytes. J Biol Chem 279:2135–2146
Stellavato A, Cirillo N (2007) Fate of desmoglein 3 in oral pemphigus vulgaris: an RT-PCR study of cell adhesion molecules. J Stomatol Invest 1:63–68
Caldelari R, de Bruin A, Baumann D, Suter MM, Bierkamp C, Balmer V, Müller E (2001) A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol 153:823–834
Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, Bolli R, Hunziker T, Suter MM, Müller EJ (2006) Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J 25:3298–3309
Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS (2006) p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci USA 103:12855–12860
Nguyen VT, Chernyavsky AI, Arredondo J, Bercovich D, Orr-Urtreger A, Vetter DE, Wess J, Beaudet AL, Kitajima Y, Grando SA (2004) Synergistic control of keratinocyte adhesion through muscarinic and nicotinic acetylcholine receptor subtypes. Exp Cell Res 294:534–549
Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA (2007) Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J Biol Chem 282:13804–13812
Chernyavsky AI, Arredondo J, Piser T, Karlsson E, Grando SA (2008) Differential coupling of M1 muscarinic and alpha7 nicotinic receptors to inhibition of pemphigus acantholysis. J Biol Chem 283:3401–3408
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2009_139_MOESM1_ESM.tif
Schematic representation of the desmosome and skin disease related to failure of desmosomal components (Fig. S1, TIFF 428 kb)
Rights and permissions
About this article
Cite this article
Cirillo, N., Prime, S.S. Desmosomal interactome in keratinocytes: a systems biology approach leading to an understanding of the pathogenesis of skin disease. Cell. Mol. Life Sci. 66, 3517–3533 (2009). https://doi.org/10.1007/s00018-009-0139-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-009-0139-7