Abstract.
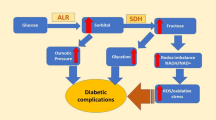
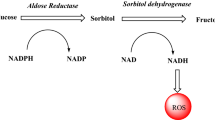
Aldose reductase and aldehyde reductase belong to the aldo-keto reductase superfamily of enzymes whose members are responsible for a wide variety of biological functions. Aldose reductase has been identified as the first enzyme involved in the polyol pathway of glucose metabolism which converts glucose into sorbitol. Glucose over-utilization through the polyol pathway has been linked to tissue-based pathologies associated with diabetes complications, which make the development of a potent aldose reductase inhibitor an obvious and attractive strategy to prevent or delay the onset and progression of the complications. Structural studies of aldose reductase and the homologous aldehyde reductase in complex with inhibitor were carried out to explain the difference in the potency of enzyme inhibition. The aim of this review is to provide a comprehensive summary of previous studies to aid the development of aldose reductase inhibitors that may have less toxicity problems than the currently available ones.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 4 December 2006; received after revision 12 February 2007; accepted 20 April 2007
Rights and permissions
About this article
Cite this article
El-Kabbani, O., Podjarny, A. Selectivity determinants of the aldose and aldehyde reductase inhibitor-binding sites. Cell. Mol. Life Sci. 64, 1970–1978 (2007). https://doi.org/10.1007/s00018-007-6514-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-007-6514-3