Abstract.
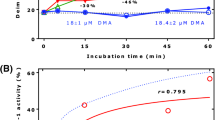
An increase in circulating asymmetric dimethylarginine (ADMA) and a decreased L-arginine/ADMA ratio are associated with reduced endothelial nitric oxide (NO) production and increased risk of vascular disease. We explored relations between ADMA, L-arginine and dimethylarginine dimethylaminohydrolase (DDAH) in liver (HepG2) cells. DDAH is the principle enzyme for the metabolism of ADMA. HepG2 cells metabolised 44.8 nmol/h of ADMA per 3.6 × 106 cells in the absence of L-arginine. The metabolism of ADMA at physiological (1μ mol/l, p < 0.01) and at pathological (100μmol/l, p < 0.01) levels was inhibited dose-dependently by L-arginine (0–400μmol/l) in cultured HepG2 cells and increased intracellular ADMA (p = 0.039). L-arginine competitively inhibited DDAH enzyme activity to 5.6 ± 2.0% of the untreated level (p < 0.01). We conclude that L-arginine regulates ADMA metabolism dose-dependently by competing for DDAH thus maintaining the metabolic balance of L-arginine and ADMA, and endothelial NO homeostasis.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 9 June 2006; received after revision 16 July 2006; accepted 19 September 2006
Rights and permissions
About this article
Cite this article
Wang, J., Sim, A.S., Wang, X.L. et al. L-arginine regulates asymmetric dimethylarginine metabolism by inhibiting dimethylarginine dimethylaminohydrolase activity in hepatic (HepG2) cells. Cell. Mol. Life Sci. 63, 2838–2846 (2006). https://doi.org/10.1007/s00018-006-6271-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-006-6271-8