No Abstract.
.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Years ago, in 1940, when I was completing my PhD thesis, the names of Embden, Meyerhof, Parnass, Cori and others were becoming immortalized in the history of biochemistry. Each had provided their piece of the jigsaw puzzle which, when put together, created the first metabolic pathway-GLYCOLYSIS.

65 years later I can look back and realize with amazement that nearly everything we know today about biochemistry and molecular biology has been discovered in my conscious scientific lifetime.
Education
My academic career at school and at Huddersfield. Technical College was “inauspicious” and by the time I was 18 I had “repeated two years” — a euphemism for having failed my first two major public examinations. My Final B.Sc.examination for a London External degree in Chemistry was “successful” but only just. This was a highly unconventional introduction to a future lifetime at a University and it left me with a deep concern for teaching, and a lasting empathy with the less illustrious student, of whom I had been one 1936 was a very difficult time for jobs — particularly for a graduate with a minimal degree, but I was lucky. The Head of the Chemistry Department at Huddersfield Technical College, Dr Hodgson, had for years had a close research relationship with the British Dyestuffs Corporation — a very large near-by branch of ICI. I guess he suspected that I had a greater potentiality than I had hitherto demonstrated because he suggested that I might be interested in research work, leading hopefully to a PhD. The downside would be years of poverty, but this could be limited if I also taught part-time — day and nights. I had had no training in teaching but in the philosophy of the time I had a degree, and therefore I could teach. So I taught — Bakery Science at first and more advanced chemistry later. Much of my research work was on aromatic nitroso and fluorine compounds — which proved to be an exciting and stimulating time. Fluorine compounds had to be made using hydrofluoric acid which necessitated the use of lead vessels in which invisible reactions frequently resulted in explosions of varying severity. A lasting memory of this time is the casual way in which we would “handle” nitroso naphthylamines now regarded as highly carcinogenic, but the word carcinogen had not then been invented. It was a wonderful four years which resulted in nine publications, and my PhD viva proved to be the first public examination I had ever passed first time. It was hopefully the start of a rather more glorious future.
Earliest chemotherapy
1941 was a traumatic time in British history and I became a works chemist in Nottingham with a large pharmaceutical firm — Boots Pure Drug Company. I was given the huge and exciting challenge of developing the large-scale manufacture of Sulphanilamide, the first chemotherapeutic drug to be produced commercially for combating pathogenic bacteria. It was wartime, and increasing casualties were creating an increasingly urgent need. Other, more efficient, sulphonamides soon followed — Sulphapyridine (the famous M&B693) active against the deadly pneumococcus, and Sulphathiazole. Towards the end of the war the company built a huge incubator to grow, very inefficiently, a mould called penicillium, to produce an “antibiotic” called penicillin. The age of chemotherapy had truly arrived and, in our different ways, we all felt it a privilege to be part.
Medical School
After the war ICI tried to encourage scientists working in industry to interrelate with the universities by founding a number of ICI Research Fellowships, one of which was in Chemotherapy at the Medical School in Leeds. Though I was well qualified in chemistry my knowledge of therapy was minimal, but I applied and was successful. My first days in the Department of Bacteriology were unforgettable and introduced me to a new life far removed from that of an organic chemist. My lab was adjacent to the diagnostic lab. with its constant stream of diphtheria throat swabs, tins of faeces or tuberculous sputum, blood samples, VD specimens, and big bottles of 24-hour urines. Professor Macleod, a delightful, very Scottish scot, started me on two “chemical” projects — the growth requirements of the tubercle bacillus (which grew very slowly), and the chemical nature of diphtheria toxins — an organism on which he was a world authority. This immediately appealed to me because in early childhood I had had both tuberculosis and diphtheria. For my first year I also became a student again, attending lectures and practical classes in bacteriology. I had to learn fast. When my research fellowship expired I became a Lecturer in Bacteriology and was required to teach mainly science students studying for a B.Sc.in Bacteriology, and occasionally the medical students. The late-1940s and 50’s were uniquely fascinating times to be learning and teaching Bacterial Metabolism, for bacteria were the major source of much of this enlightenment. Bacteria were ideal organisms for revealing the chemical nature of genetics as Avery, Macleod and McCarty in 1944 had discovered by showing the ability of DNA to transfer genetic material in pneumococci Two years later Lederberg and Tatum had reported one of the most exciting discoveries of all — that of conjugation between some bacteria as a means of transmitting genetic material from a donor bacterium to a recipient — from a male (sometimes a “super male”) to a female. Their interrupted mating experiment was a major contribution to a growing understanding of gene sequences and of the transfer of DNA, which led ultimately to the elucidation of its structure by Crick and Watson in 1953.
Metabolic Pathways Charts
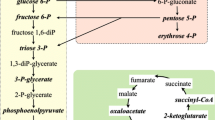
At the same time as these investigations on the genetics of bacteria were evolving, an explosion in our understanding of the chemical nature of living organisms was also taking place The first metabolic pathway, Glycolysis been completed around 1940 and this had stimulated research into other pathways to such an extent that, by the mid-1950s, dozens had been described. This created a growing problem for a teacher of Bacterial Metabolism. Students would memorise a lot of pathways and structures and enzymes, but could not put them together to make it all meaningful. Some pathways were clearly related to each other but for an organisms to live it was necessary for them all to be integrated into a living concerto. The situation was akin to an unmade jigsaw puzzle which would only become fully meaningful when all the pieces had been put together — a process greatly simplified if accompanied by its picture. My challenge was to design an integrated picture of metabolic pathways that would be able to complement textbooks by providing an over-all picture of metabolism and encourage an understanding and appreciation of the significance and elegance of interrelated pathways. My first Metabolic Pathways Chart was created in 1955 — just 50 years ago. They were handdrawn by stencils on tracing paper and “blue-printed” in the University architect’s department and distributed to colleagues in the Department of Biochemistry who knew much more biochemistry that I did. Their response was immediate and generally enthusiastic and when a few maps filtered down to students the demand grew. During the next few years two new updated maps were produced but logistic problems became so acute that a search for a proper publisher became essential. This took months until, in 1960, I had an enthusiastic response from a small biochemical firm, Koch-Light Laboratories — and soon the first printed Metabolic Pathways Chart was born. It was designed to show the integration of amino acid, carbohydrate, lipid and other pathways, which were identified and differentiated by colour, and were linked to a central backbone of glycolysis, the citric acid cycle, and the respiratory chain and (very much later) the nanomotor ATP synthase. This was the start of a long and fruitful relationship which in the next 28 years produced 18 editions and 800,000 copies and were incorporated in a dozen books including prestigious biochemistry text books From the very beginning I had inestimable encouragement from many sources especially Stan Dagley with whom I later the wrote what became the standard text — An Introduction to Metabolic Pathways. My greatest surprise and satisfaction came from the responses of the Professors of Biochemistry at both Oxford and Cambridge. Rudolph Peters at Cambridge asked to use one of my earliest maps in a prestigious lecture and in a book. The Professor at Oxford was Hans Krebs. I well remember going to hear him lecture on Metabolic Interrelationships and his first slide and the basis of his lecture was my new map. He would regularly acknowledge subsequent editions and enthusiastically responded to the advent of An Introduction to Metabolic Pathways. Later, at the FEBS meeting in Prague in 1968 he asked me if I would edit the new Volume 4 of the prestigious Geigy Scientific Tables for which he had hitherto been responsible. Such confidence from such a source did wonders for my morale.
Inborn Errors of Metabolism
The Bacteriology Department in those days was part of the Medical School and I got to know some of the medical students well. Many felt that while bacteriology was clearly an acceptable part of their curriculum, biochemistry was not. My metabolic pathways chart did not always help and could sometimes induce a dis-ease which I called the “O-Hell Syndrome” — a phobia caused by the fear that it might have to be memorized. Could it be revised so as to become acceptable, or even an inspiration, to a choosy medical student? If a gene is defective it may result in a defective enzyme in part of a metabolic pathway, and this could result in the concentration of its substrate — usually in the blood or urine. More than a hundred such potentially deficient enzymes existed on my Metabolic Pathways Chart and could be linked with the known diseases for which they were responsible. The Inborn Errors of Metabolism map was thus created to encourage medical students to realize that an understanding of biochemistry and genetics could be an important asset in their understanding of clinical medicine In 1990 Koch-Light Laboratories, which had published my maps for 28 years, was taken over and I had, sadly, to look elsewhere for a publisher I had a good friend who was a director of the UK branch of the international Sigma Chemical Company, and he suggested that I rang up the President of the company in St Louis. With considerable trepidation I did just this and to my great surprise and appreciation we talked for 20 minutes. Tom Cori, the founder and President of Sigma (now Sigma-Aldrich), has a unique biochemical pedigree. Both his parents were Nobel laureates and some of their work stands at the very centre of my maps and of biochemistry itself. My relationship with Sigma over the last 15 years has been exciting and creative, resulting in maps which are not only attractive and useful, but also works of art. The biggest step forward arose when the maps were digitised. This was a new and wonderful word to me because it meant that — never again would I have to create a map by sticking thousands of little bits of paper on a big piece of cardboard. It was all on computer, and future editions would be relatively easy to make.
Minimaps and Animaps
As I grew older I became increasing concerned about the future of the maps. They had been part of my life and of the biochemical world for 40 years and I was concerned about their perpetuation, so I approached the International Union of Biochemistry and Molecular Biology and offered them the copyright of all my work, an offer which was enthusiastically accepted. At the same time a new and exciting development was gestating. For years I had wanted to modernise the concept of the small maps which were the basis of the Introduction to Metabolic Pathways of 25 years earlier, so on my 80th birthday, I bought my first computer and the Minimaps were born. Each minimap is a colour picture of a separate pathway and includes co-factors, regulation, compartmentation and other features to illustrate their significance within the cell. Some are specifically medically oriented — again to encourage medical students, but all are, as far as possible, designed to relate to “real life”. An example of this is the minimap “Products of Isoprene Metabolism” shown in figure 2 which illustrates the exciting variety of products which derive from the same, simple 5-carbon compound — isoprene. They include natural rubber, plant and insect hormones, and the side chains of many vitamins and of chlorophyll. They provide the colour, smell and flavour of many plant products such as carrots, lemons and roses. They include some of the vital reactions that convert light into vision, and, in a different pathway, the steroids including estrogens and testosterones with all their implications. Best of all, with the growth of the Internet they could all be downloaded freely throughout the world on www.iubmb.org. The response to the minimaps has been vast, but a basic drawback remained unresolved. They were just static portrayals of what are dynamic processes. Biochemistry is a living subject, BIO-chemistry, and to make it meaningful, metabolism had to be animated. Minimaps had to become animaps. The first animaps were created three years ago using PowerPoint. I was able to show the flow of reactants into the inside of the cell where they would align with the active sites within the cell prior to reaction — but I could not show the reactions themselves. Movement of bonds, traditionally illustrated by curly arrow, required a swivelling motion which PowerPoint could not do. So I turned to Flash, which made possible a unique visual representation of bond and electron movements. A lone pair of electrons on a nitrogen atom or a negative sign on an oxygen can each be seen to become bonds and thus bring a new dimension into the visualisation of organic chemistry as well as biochemistry. My immediate objective now is to complete the animation of the sequence of reactions which starts with glucose and continues through the citric acid cycle and the respiratory chain to ATP synthase — a sequence which is the basis for the provision of energy for nearly all anabolic reactions. The first metabolic pathway to be thus animated was glycolysis. The citric acid cycle was animated in a rather different way — to clarify its purpose — the harnessing of the potential energy of acetyl-CoA into NADH and UQH2 — a purpose which is rarely clearly illustrated in textbooks. The most exciting of the Flash animations so far is that of ATP synthase, which illustrates how retrolocating protons drive the molecular motor that synthesises ATP. It stands at the very heart of metabolism — between the breakdown products of metabolism and their re-creation — between catabolism and anabolism. It is the heart of metabolism and is so vital that it is calculated that we synthesise (and degrade) our body’s weight of ATP every day. It is hoped that such an animation will stimulate an appreciation that although of very different architecture, in elegance and beauty and in biochemical significance it is akin to DNA.
I am now nearly 90 and can only look back in amazement at the magnitude and range of our understanding of cellular and molecular life sciences — and wonder what is the contribution of the teacher. It is not merely to assimilate and interpret the work of others but, at a more basic level, at the very beginning, to encourage and inspire an excitement that seeks for fuller understanding, for enlightenment. And what can be more exciting than the science of life? I have had a wonderful life and have received thousands of (mostly) enthusiastic messages of appreciation, especially from students. One of the most gratifying was to receive a book written by a former student who has had a very successful career in the media. It was inscribed simply: “To a teacher that made the difference”. All the work here described has been aimed to “make the difference” and the most satisfying achievement of that aspiration is that everything that I have here described is now freely available to all, however impoverished, throughout the world — on the internet — on www.iubmb.org. Over the years my aim, and more recently my aphorism, has been “TO MAKE METABOLISM MEANINGFUL, WONDER-FULL, — AND FUN”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 18 October 2005; accepted 18 October 2005
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nicholson, D. A Lifetime of Metabolism. Cell. Mol. Life Sci. 63, 1–5 (2006). https://doi.org/10.1007/s00018-005-5500-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-005-5500-x