Abstract
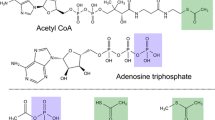
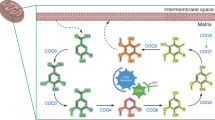
Acetyl-coenzyme A synthetase (AMP forming; Acs) is an enzyme whose activity is central to the metabolism of prokaryotic and eukaryotic cells. The physiological role of this enzyme is to activate acetate to acetyl-coenzyme A (Ac-CoA). The importance of Acs has been recognized for decades, since it provides the cell the two-carbon metabolite used in many anabolic and energy generation processes. In the last decade researchers have learned how carefully the cell monitors the synthesis and activity of this enzyme. In eukaryotes and prokaryotes, complex regulatory systems control acs gene expression as a function carbon flux, with a second layer of regulation exerted posttranslationally by the NAD+/sirtuin-dependent protein acetylation/deacetylation system. Recent structural work provides snapshots of the dramatic conformational changes Acs undergoes during catalysis. Future work on the regulation of acs gene expression will expand our understanding of metabolic integration, while structure/function studies will reveal more details of the function of this splendid molecular machine.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 4 December 2003; received after revision 2 March 2004; accepted 16 March 2004
Rights and permissions
About this article
Cite this article
Starai, V.J., Escalante-Semerena, J.C. Acetyl-coenzyme A synthetase (AMP forming). CMLS, Cell. Mol. Life Sci. 61, 2020–2030 (2004). https://doi.org/10.1007/s00018-004-3448-x
Issue Date:
DOI: https://doi.org/10.1007/s00018-004-3448-x