Abstract
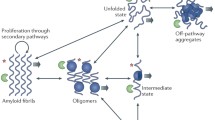
The formation of amyloid fibrils is associated with several devastating diseases in humans and animals, including e.g. Alzheimer’s disease (AD) and the spongiform encephalopathies. Here, we review and discuss the current knowledge on two amyloid peptides: lung surfactant protein C (SP-C) and the amyloid β-peptide (Aβ), implicated in human lung disease and in AD, respectively. Both these hydrophobic peptides are derived from the transmembrane region of their precursor protein, and can transit from a monomeric α-helical state to a β-sheet fibril. The α helices of SP-C and Aβ are composed of amino acid residues with inherently higher propensities for β strand than helix conformation. Their helical states are stabilized by a membrane environment, and loss of membrane association thus promotes structural conversion and fibril formation. We speculate that the loss of structural context for sequences with a high propensity for formation of β sheets may be a common feature of amyloid formation in general.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 9 July 2003; received after revision 15 August 2003; accepted 3 September 2003
Rights and permissions
About this article
Cite this article
Johansson, J., Weaver, T.E. & Tjernberg, L.O. Proteolytic generation and aggregation of peptides from transmembrane regions: lung surfactant protein C and amyloid β-peptide. CMLS, Cell. Mol. Life Sci. 61, 326–335 (2004). https://doi.org/10.1007/s00018-003-3274-6
Issue Date:
DOI: https://doi.org/10.1007/s00018-003-3274-6