Abstract
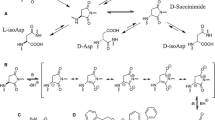
Formation of β-linked Asp-Xaa peptide bonds – isoaspartyl (isoAsp) sites – arise in proteins via succinimide−linked deamidation of asparagine or dehydration of aspartate, reactions which represent a major source of spontaneous protein damage under physiological conditions. Accumulation of atypical isoaspartyl sites is minimized in vivo by the activity of protein L-isoaspartyl O–methyltransferase (PIMT), which regenerates a normal peptide bond. Loss of PIMT has harmful consequences, especially in neurons; thus, formation of isoAsp sites and their subsequent correction by PIMT is widely believed to constitute an important pathway of protein damage and repair. Recent evidence is mounting, however, that deamidation and isoaspartate formation may, in some instances, constitute a novel mechanism for intentional modification of protein structure. Herein we describe the mechanism of Asx rearrangement, summarize the evidence that PIMT serves an important repair function, and then focus on emerging evidence that deamidation and isoAsp formation may sometimes have a useful function.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 16 October 2002; received after revision 11 December 2002; accepted 12 December 2002
Rights and permissions
About this article
Cite this article
Reissner, K.J., Aswad, D.W. Deamidation and isoaspartate formation in proteins: unwanted alterations or surreptitious signals?. CMLS, Cell. Mol. Life Sci. 60, 1281–1295 (2003). https://doi.org/10.1007/s00018-003-2287-5
Issue Date:
DOI: https://doi.org/10.1007/s00018-003-2287-5