Abstract
Objectives
We aimed at identifying the role of transient receptor potential (TRP) channels in pterygium.
Methods
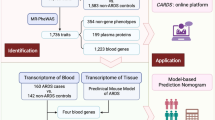
Based on microarray data GSE83627 and GSE2513, differentially expressed genes (DEGs) were screened and 20 hub genes were selected. After gene correlation analysis, 5 TRP-related genes were obtained and functional analyses of gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were performed. Multifactor regulatory network including mRNA, microRNAs (miRNAs) and transcription factors (TFs) was constructed. The 5 gene TRP signature for pterygium was validated by multiple machine learning (ML) programs including support vector classifiers (SVC), random forest (RF), and k-nearest neighbors (KNN). Additionally, we outlined the immune microenvironment and analyzed the candidate drugs. Finally, in vitro experiments were performed using human conjunctival epithelial cells (CjECs) to confirm the bioinformatics results.
Results
Five TRP-related genes (MCOLN1, MCOLN3, TRPM3, TRPM6, and TRPM8) were validated by ML algorithms. Functional analyses revealed the participation of lysosome and TRP-regulated inflammatory pathways. A comprehensive immune infiltration landscape and TFs-miRNAs-mRNAs network was studied, which indicated several therapeutic targets (LEF1 and hsa-miR-455-3p). Through correlation analysis, MCOLN3 was proposed as the most promising immune-related biomarker. In vitro experiments further verified the reliability of our in silico results and demonstrated that the 5 TRP-related genes could influence the proliferation and proinflammatory signaling in conjunctival tissue contributing to the pathogenesis of pterygium.
Conclusions
Our study suggested that TRP channels played an essential role in the pathogenesis of pterygium. The identified pivotal biomarkers (especially MCOLN3) and pathways provide novel directions for future mechanistic and therapeutic studies for pterygium.








Similar content being viewed by others
Data availability
All data can be obtained from the corresponding authors under reasonable request.
References
Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ Open. 2013;3: e003787.
Zhao Z, Zhang J, Liang H, et al. Corneal reinnervation and sensitivity recovery after pterygium excision. J Ophthalmol. 2020;2020:1–8.
Van Acker SI, Van den Bogerd B, Haagdorens M, et al. Pterygium—the good, the bad, and the ugly. Cells. 2021;10:1567.
Chui J, Coroneo MT, Tat LT, et al. Ophthalmic pterygium. Am J Pathol. 2011;178:817–27.
Artornsombudh P, Sanpavat A, Tinnungwattana U, et al. Prevalence and clinicopathologic findings of conjunctival epithelial neoplasia in pterygia. Ophthalmology. 2013;120:1337–40.
Ghiasian L, Samavat B, Hadi Y, Arbab M, Abolfathzadeh N. Recurrent pterygium: a review. J Curr Ophthalmol. 2021;33:367.
Chu WK, Choi HL, Bhat AK, Jhanji V. Pterygium: new insights. Eye. 2020;34:1047–50.
Bradley JC, Yang W, Bradley RH, Reid TW, Schwab IR. The science of pterygia. Br J Ophthalmol. 2010;94:815–20.
Nilius B, Voets T, Peters J. TRP channels in disease. Sci STKE. 2005;2005(295):8.
Wang H, Cheng X, Tian J, et al. TRPC channels: structure, function, regulation and recent advances in small molecular probes. Pharmacol Ther. 2020;209: 107497.
Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–84.
Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218.
Khajavi N, Reinach PS, Slavi N, et al. Thyronamine induces TRPM8 channel activation in human conjunctival epithelial cells. Cell Signal. 2015;27:315–25.
Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investig Opthalmology Vis Sci. 2011;52:485.
Hirata H, Oshinsky ML. Ocular dryness excites two classes of corneal afferent neurons implicated in basal tearing in rats: involvement of transient receptor potential channels. J Neurophysiol. 2012;107:1199–209.
Trusiano B, Tupik JD, Allen IC. Cold sensor, hot topic: TRPM8 plays a role in monocyte function and differentiation. J Leukoc Biol. 2022;112:361.
Assimakopoulou M, Pagoulatos D, Nterma P, Pharmakakis N. Immunolocalization of cannabinoid receptor type 1 and CB2 cannabinoid receptors, and transient receptor potential vanilloid channels in pterygium. Mol Med Rep. 2017;16:5285–93.
Goecks J, Jalili V, Heiser LM, Gray JW. How machine learning will transform biomedicine. Cell. 2020;181:92–101.
Ye B, Liu K, Cao S, et al. Discrimination of indoor versus outdoor environmental state with machine learning algorithms in myopia observational studies. J Transl Med. 2019;17:314.
An G, Omodaka K, Tsuda S, et al. Comparison of machine-learning classification models for glaucoma management. J Healthc Eng. 2018;2018:1–8.
Yan H, Shan X, Wei S, Liu F, Li W, Lei Y, et al. Abnormal spontaneous brain activities of limbic-cortical circuits in patients with dry eye disease. Front Hum Neurosci. 2020;14: 574758.
Nwanosike EM, Conway BR, Merchant HA, Hasan SS. Potential applications and performance of machine learning techniques and algorithms in clinical practice: a systematic review. Int J Med Inf. 2022;159: 104679.
Banna HU, Zanabli A, McMillan B, et al. Evaluation of machine learning algorithms for trabeculectomy outcome prediction in patients with glaucoma. Sci Rep. 2022;12:2473.
Sugimoto K, Murata H, Hirasawa H, et al. Cross-sectional study: does combining optical coherence tomography measurements using the ‘Random Forest’ decision tree classifier improve the prediction of the presence of perimetric deterioration in glaucoma suspects? BMJ Open. 2013;3: e003114.
Muhammad H, Fuchs TJ, De Cuir N, et al. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. J Glaucoma. 2017;26:1086–94.
Joshi VS, Maude RJ, Reinhardt JM, et al. Automated detection of malarial retinopathy-associated retinal hemorrhages. Investig Opthalmol Vis Sci. 2012;53:6582.
Kishore B, Ananthamoorthy NP. Glaucoma classification based on intra-class and extra-class discriminative correlation and consensus ensemble classifier. Genomics. 2020;112:3089–96.
Zheng J. Molecular mechanism of TRP channels. Compr Physiol. 2013;3(1):221–42.
Venkatachalam K, Montell C. TRP Channels. Annu Rev Biochem. 2007;76:387–417.
Wu N, Yan C, Chen J, et al. Conjunctival reconstruction via enrichment of human conjunctival epithelial stem cells by p75 through the NGF-p75-SALL2 signaling axis. Stem Cells Transl Med. 2020;9:1448.
Wu N, Gong D, Chen J, et al. Design of functional decellularized matrix for conjunctival epithelial stem cell maintenance and ocular surface reconstruction. Mater Des. 2022;224: 111278.
Arora S, Rana R, Chhabra A, Jaiswal A, Rani V. miRNA–transcription factor interactions: a combinatorial regulation of gene expression. Mol Genet Genom. 2013;288:77–87.
Golu T, Mogoant L, Streba CT, Pirici DN. Pterygium: histological and immunohistochemical aspects. Rom J Morphol Embryol. 2011;52(1):153–8.
Tekelioglu Y, Turk A, Avunduk AM, Yulug E. Flow cytometrical analysis of adhesion molecules, T-lymphocyte subpopulations and inflammatory markers in pterygium. Ophthalmologica. 2006;220:372–8.
Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23:195–228.
Bianchp E, Grande C, Platerotp R, et al. Immunohistochemical profile of VEGF, TGF-β and PGE2 in human pterygium and normal conjunctiva: experimental study and review of the literature. Int J Immunopathol Pharmacol. 2012;25(3):607–15.
Kim SW, Kim H-I, Thapa B, Nuwormegbe S, Lee K. Critical role of mTORC2-Akt signaling in TGF-β1-induced myofibroblast differentiation of human pterygium fibroblasts. Investig Opthalmol Vis Sci. 2019;60:82.
Xie J, Ning Q, Zhang H, Ni S, Ye J. RhoA/ROCK signaling regulates TGF-β1-Induced fibrotic effects in human pterygium fibroblasts through MRTF-A. Curr Eye Res. 2022;47:196–205.
Vrenken KS, Jalink K, van Leeuwen FN, Middelbeek J. Beyond ion-conduction: channel-dependent and -independent roles of TRP channels during development and tissue homeostasis. Biochim Biophys Acta BBA Mol Cell Res. 2016;1863:1436–46.
Gan N, Han Y, Zeng W, Wang Y, Xue J, Jiang Y. Structural mechanism of allosteric activation of TRPML1 by PI(3,5)P2 and rapamycin. Proc Natl Acad Sci. 2022;119: e2120404119.
Jaslan D, Böck J, Krogsaeter E, Grimm C. Evolutionary aspects of TRPMLs and TPCs. Int J Mol Sci. 2020;21:4181.
Li G, Huang D, Li N, Ritter JK, Li P-L. Regulation of TRPML1 channel activity and inflammatory exosome release by endogenously produced reactive oxygen species in mouse podocytes. Redox Biol. 2021;43:102013.
Wakabayashi K, Gustafson AM, Sidransky E, Goldin E. Mucolipidosis type IV: an update. Mol Genet Metab. 2011;104:206–13.
Noben-Trauth K. The TRPML3 channel: from gene to function. Adv Exp Med Biol. 2011;704:229–37.
Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161:1306–19.
Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca2+ channel TRPML3 regulates membrane trafficking and autophagy. Traffic Cph Den. 2009;10:1157–67.
Zierler S. TRPM channels as potential therapeutic targets against pro-inflammatory diseases. Cell Calcium. 2017;67:105.
Held K, Voets T, Vriens J. TRPM3 in temperature sensing and beyond. Temp Austin Tex. 2015;2:201–13.
Takashina Y, Manabe A, Hasegawa H, et al. Sodium citrate increases expression and flux of Mg2+ transport carriers mediated by activation of MEK/ERK/c-Fos pathway in renal tubular epithelial cells. Nutrients. 2018;10:E1345.
Yang F, Cai J, Zhan H, et al. Suppression of TRPM7 inhibited hypoxia-induced migration and invasion of androgen-independent prostate cancer cells by enhancing RACK1-Mediated degradation of HIF-1α. Oxid Med Cell Longev. 2020;2020:6724810.
Khalil M, Babes A, Lakra R, et al. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol. 2016;9:1500–13.
Chui J, Girolamo ND, Wakefield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6:24–43.
Anguria P, Carmichael T, Ntuli S, Kitinya J. Chronic inflammatory cells and damaged limbal cells in pterygium. Afr Health Sci. 2013;13:725–30.
Garreis F, Schroder A, Reinach PS, et al. Upregulation of transient receptor potential vanilloid Type-1 channel activity and and Ca2+ influx dysfunction in human pterygial cells. Invest Ophthalmol Vis Sci. 2016;57(6):2564–77.
Sumioka T, Okada Y, Reinach PS, et al. Impairment of corneal epithelial wound healing in a TRPV1-deficient mouse. Investig Opthalmol Vis Sci. 2014;55:3295.
Hobert O. Common logic of transcription factor and microRNA action. Trends Biochem Sci. 2004;29:462–8.
Ghafouri-Fard S, Abak A, Fattahi F, et al. The interaction between miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders. Biomed Pharmacother Biomed Pharmacother. 2021;138: 111519.
Ueta M, Nishigaki H, Sotozono C, et al. Regulation of gene expression by miRNA-455-3p, upregulated in the conjunctival epithelium of patients with Stevens-Johnson syndrome in the chronic stage. Sci Rep. 2020;10:17239.
Kato N, Shimmura S, Kawakita T, et al. β-Catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Investig Opthalmol Vis Sci. 2007;48:1511.
Huebner K, Procházka J, Monteiro AC, Mahadevan V, Schneider-Stock R. The activating transcription factor 2: an influencer of cancer progression. Mutagenesis. 2019;34:375–89.
Katoh M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci. 2019;133:953–70.
Principe DR. Patients deriving long-term benefit from immune checkpoint inhibitors demonstrate conserved patterns of site-specific mutations. Sci Rep. 2022;12:11490.
Tong L, Lan W, Lim RR, Chaurasia SS. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul Surf. 2014;12:23–31.
Maxia C, Murtas D, Corrias M, et al. Vitamin D and vitamin D receptor in patients with ophthalmic pterygium. Eur J Histochem. 2017;61(4):2837.
Skurikhin E, Pershina O, Zhukova M, et al. Spiperone stimulates regeneration in pulmonary endothelium damaged by cigarette smoke and lipopolysaccharide. Int J Chron Obstruct Pulmon Dis. 2021;16:3575–91.
Skurikhin E, Madonov P, Pershina O, et al. Micellar hyaluronidase and spiperone as a potential treatment for pulmonary fibrosis. Int J Mol Sci. 2021;22:5599.
Kistemaker LEM, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci. 2015;36:164–71.
Yang Q, Jhanji V, Tan SQ, et al. Continuous exposure of nicotine and cotinine retards human primary pterygium cell proliferation and migration. J Cell Biochem. 2019;120:4203–13.
Acknowledgements
We would like to express our sincere gratitude to the GEO database for sharing the transcriptome data. We also thank the Shanghai Key Laboratory of Orbital Diseases and Ocular Oncology for providing the research platform.
Funding
This work was supported by the National Natural Science Foundation of China (No.81770888 and 82271041).
Author information
Authors and Affiliations
Contributions
YC: data interpretation, in vitro experiment, and manuscript writing. TZ: machine learning and in vitro experiment. JC: manuscript revision. XC: study design, data collection and interpretation. YF: manuscript revision and study supervision. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest is declared.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Y., Zhou, T., Chen, J. et al. Uncovering the role of transient receptor potential channels in pterygium: a machine learning approach. Inflamm. Res. 72, 589–602 (2023). https://doi.org/10.1007/s00011-023-01693-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01693-4