Abstract
Background
Activation of intestinal macrophages is implicated in the pathogenesis of neonatal necrotizing enterocolitis (NEC), yet its precise mechanisms remain unclear.
Objective
The purpose of this study is to investigate the role of macrophages and TNF-α via an inflammatory MicroRNA in NEC.
Materials and methods
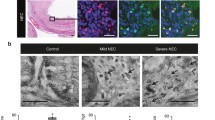
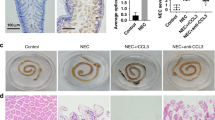
Immunofluorescence (IF) staining of CD68, iNOS, and Arg-1 was employed to identify phenotypes of macrophage in the intestines of NEC infants and NEC mice. Expression of TNF-α, c-kit, and miR-222 was evaluated by qRT-PCR, Western blot, and immunochemical staining from the tissue samples.
Results
Large number of M1 macrophage infiltration was found in the NEC intestines. Expression of CD68, iNOS, and TNF-α were significantly increased, while c-kit was decreased distinctly in the NEC group. In the early phase of NEC mouse model, inhibition of M1 macrophages reduced the incidence of NEC and intestinal inflammation. We found that TNF-α upregulated the expression of miRNA-222 and inhibited the expression of c-kit. Conversely, such decrease of c-kit expression could be reversed by miR-222 antagonists. Furtherly, dual-luciferase assay confirmed that c-kit can be inhibited by miR-222 directly.
Conclusion
Macrophages activation in NEC intestine results in an increased inflammatory response and TNF-α production, accompanied with miR-222 upregulation and c-kit suppression. Modulations of M1 macrophages, TNF-α or miR-222 may be potential therapeutic targets for NEC treatment.






Similar content being viewed by others
Abbreviations
- Arg-1:
-
Arginase 1
- CL:
-
Liposomal clodronate
- DAPI:
-
4,6-Diamidino-2-phenylindole
- ELAM-1:
-
Endothelial-leukocyte adhesion molecule 1
- FBS:
-
Fetal bovine serum
- HE:
-
Hematoxylin and eosin
- ICCs:
-
Interstitial cells of Cajal
- IF:
-
Immunofluorescence
- IFN:
-
Interferon
- iNOS:
-
Inducible NO synthase
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- NF-kB:
-
Nuclear factor kappa light chain enhancer of activated B cells
- NEC:
-
Neonatal necrotizing enterocolitis
- NS:
-
Normal saline
- PAF:
-
Platelet activating factor
- SCF:
-
Stem cell factor
- SPF:
-
Specific pathogen free
- TNF:
-
Tumor necrosis factor
- TNFR1:
-
TNF receptor 1
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Lim JC, Golden JM, Ford HR. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int. 2015;31(6):509–18. https://doi.org/10.1007/s00383-015-3697-9.
Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. 2011;16(3):145–50. https://doi.org/10.1016/j.siny.2011.02.002.
MohanKumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G93-102. https://doi.org/10.1152/ajpgi.00016.2012.
Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care. 2012;12(2):77–87. https://doi.org/10.1097/ANC.0b013e31824cee94 (quiz 8-9).
Wu SF, Caplan M, Lin HC. Necrotizingenterocolitis: old problem with new hope. Pediatr Neonatol. 2012;53:158–63.
Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin Perinatol. 2012;39(2):387–401. https://doi.org/10.1016/j.clp.2012.04.011.
Xia X, Feng J. The role of TLR4 in neonatal necrotizing enterocolitis. Chinese J Pediatr Surg. 2013;34:947–50.
Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94(3):859–907. https://doi.org/10.1152/physrev.00037.2013.
Rolle U, Piaseczna-Piotrowska A, Puri P. Interstitial cells of Cajal in the normal gut and in intestinal motility disorders of childhood. Pediatr Surg Int. 2007;23(12):1139–52. https://doi.org/10.1007/s00383-007-2022-7.
Wei J, Li N, Xia X, Chen X, Peng F, Besner GE, et al. Effects of lipopolysaccharide-induced inflammation on the interstitial cells of Cajal. Cell Tissue Res. 2014;356(1):29–37. https://doi.org/10.1007/s00441-013-1775-7.
Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, et al. HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep. 2012;28(6):2285–9. https://doi.org/10.3892/or.2012.2058.
Moore CC, McKillop IH, Huynh T. MicroRNA expression following activated protein C treatment during septic shock. J Surg Res. 2013;182(1):116–26. https://doi.org/10.1016/j.jss.2012.07.063.
Ortega FJ, Moreno M, Mercader JM, Moreno-Navarrete JM, Fuentes-Batllevell N, Sabater M, et al. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin Epigenetics. 2015;7:49. https://doi.org/10.1186/s13148-015-0083-3.
Pandis I, Ospelt C, Karagianni N, Denis MC, Reczko M, Camps C, et al. Identification of microRNA-221/222 and microRNA-323-3p association with rheumatoid arthritis via predictions using the human tumour necrosis factor transgenic mouse model. Ann Rheum Dis. 2012;71(10):1716–23. https://doi.org/10.1136/annrheumdis-2011-200803.
Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52(1):245–55. https://doi.org/10.1016/j.yjmcc.2011.11.008.
Gits CM, van Kuijk PF, Jonkers MB, Boersma AW, van Ijcken WF, Wozniak A, et al. MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. Br J Cancer. 2013;109(6):1625–35. https://doi.org/10.1038/bjc.2013.483.
Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177(5):3273–82. https://doi.org/10.4049/jimmunol.177.5.3273.
Wei J, Besner GE. M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J Surg Res. 2015;197(1):126–38. https://doi.org/10.1016/j.jss.2015.03.023.
Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G442–50. https://doi.org/10.1152/ajpgi.00182.2009.
Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103. https://doi.org/10.1615/critreveukargeneexpr.v20.i2.10.
Seitz G, Warmann SW, Guglielmetti A, Heitmann H, Ruck P, Kreis ME, et al. Protective effect of tumor necrosis factor alpha antibody on experimental necrotizing enterocolitis in the rat. J Pediatr Surg. 2005;40(9):1440–5. https://doi.org/10.1016/j.jpedsurg.2005.05.043.
Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-α. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G757–64. https://doi.org/10.1152/ajpgi.00408.2005.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. https://doi.org/10.1097/00000658-197801000-00001.
Wijnands KA, Hoeksema MA, Meesters DM, van den Akker NM, Molin DG. Arginase-1 deficiency regulates arginine concentrations and NOS2-mediated NO production during endotoxemia. PLoS ONE. 2014;9:e86135.
Quirino IE, Carneiro MB, Cardoso VN, das Dos SantosVieiraFiuza GCRLQJA, et al. Arginine supplementation induces Arginase activity and inhibits TNF-α synthesis in mice spleen macrophages after intestinal obstruction. JPEN J Parenter Enteral Nutr. 2016;40(3):417–22. https://doi.org/10.1177/0148607114546374.
Bhatia AM, Stoll BJ, Cismowski MJ, Hamrick SE. Cytokine levels in the preterm infant with neonatal intestinal injury. Am J Perinatol. 2014;31(6):489–96. https://doi.org/10.1055/s-0033-1353437.
McElroy SJ, Prince LS, Weitkamp JH, Reese J, Slaughter JC, Polk DB. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011;301(4):G656–66. https://doi.org/10.1152/ajpgi.00550.2010.
Guven A, Gundogdu G, Vurucu S, Uysal B, Oztas E, Ozturk H, et al. Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2009;44(9):1730–5. https://doi.org/10.1016/j.jpedsurg.2009.01.007.
Guven A, Uysal B, Gundogdu G, Oztas E, Ozturk H, Korkmaz A. Melatonin ameliorates necrotizing enterocolitis in a neonatal rat model. J Pediatr Surg. 2011;46(11):2101–7. https://doi.org/10.1016/j.jpedsurg.2011.06.040.
Ozdemir R, Yurttutan S, Sari FN, Uysal B, Unverdi HG, Canpolat FE, et al. Antioxidant effects of N-acetylcysteine in a neonatal rat model of necrotizing enterocolitis. J Pediatr Surg. 2012;47(9):1652–7. https://doi.org/10.1016/j.jpedsurg.2012.02.016.
Neu J. Necrotizing enterocolitis. World Rev Nutr Diet. 2014;110:253–63. https://doi.org/10.1159/000358474.
Gfroerer S, Rolle U. Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease. Pediatr Surg Int. 2013;29(9):889–97. https://doi.org/10.1007/s00383-013-3364-y.
Rumessen JJ, Vanderwinden JM, Horn T. Ulcerative colitis: ultrastructure of interstitial cells in myenteric plexus. Ultrastruct Pathol. 2010;34(5):279–87. https://doi.org/10.3109/01913121003770701.
Rumessen JJ, Vanderwinden JM, Horn T. Crohn’s disease of the colon: ultrastructural changes in submuscular interstitial cells of Cajal. Cell Tissue Res. 2011;343(2):421–8. https://doi.org/10.1007/s00441-010-1087-0.
Mikkelsen HB. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med. 2010;14(4):818–32. https://doi.org/10.1111/j.1582-4934.2010.01025.x.
Rumessen JJ, Vanderwinden JM, Horn T. Crohn’s disease: ultrastructure of interstitial cells in colonic myenteric plexus. Cell Tissue Res. 2011;344(3):471–9. https://doi.org/10.1007/s00441-011-1175-9.
Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, et al. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell. 2007;129(3):617–31. https://doi.org/10.1016/j.cell.2007.02.048.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFE0203900), the National Natural Science Foundation of China (Nos. 81873541), and Sanming Project of Medicine in Shenzhen (SZSM201812055).
Author information
Authors and Affiliations
Contributions
Each named author has substantially contributed to conducting the research and drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Animal Care and Use of the Committee at Tongji Medical College (Wuhan, China) and was performed in accordance with the ethical guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2021_1441_MOESM1_ESM.tif
Supplementary file1 Supplemental Fig. 1 Histologic scoring of intestines stained with HE in mouse NEC model. Representative examples of histologic scoring of intestine determined by HE staining as grade 0 (a, normal intestine), grade 1 (b, epithelial cell lifting or separation), grade 2 (c, necrosis to mid villus level), grade 3 (d, necrosis of entire villus), and grade 4 (e, transmural necrosis). Any injury of ≥grade 2 is considered to be consistent with NEC. Scale bar = 50 µm. Magnification: ×200. (TIF 3976 KB)
Rights and permissions
About this article
Cite this article
Xia, X., Wang, D., Yu, L. et al. Activated M1 macrophages suppress c-kit expression via TNF-α-mediated upregulation of miR-222 in Neonatal Necrotizing Enterocolitis. Inflamm. Res. 70, 343–358 (2021). https://doi.org/10.1007/s00011-021-01441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01441-6