Abstract
Objective
To elucidate if TLR4-mediated MyD88 and TRIF signalling by the clinically applicable Lipopolysaccharide (LPS)-derivative monophosphoryl lipid A (MPLA) in primary human dendritic cells requires LPS cofactors LPS-binding protein (LBP) and CD14.
Methods
Cytokine production by monocyte-derived DCs stimulated with MPLA or LPS was determined using ELISA. To investigate involvement of CD14 for action of LPS or MPLA, CD14 was inhibited using blocking antibodies or down-modulated using specific siRNA. To assess involvement of LBP monocyte-derived DCs were stimulated in serum-free culture medium in absence or presence of purified LBP.
Results
LBP and CD14 are not required for and do not enhance the capacity of MPLA to induce MyD88- and TRIF-dependent pro-inflammatory IL-6 and TNF-α. Interestingly, although CD14 is required for TRIF-dependent downstream events in mice, we show that in human CD14 is redundant for MPLA-induced TRIF-dependent chemokine production.
Conclusions
These findings provide novel insight in the modes of action of MPLA in human and show that, compared to LPS, MyD88 and TRIF signalling in dendritic cells by MPLA is not mediated nor amplified by TLR4 cofactors. This gives insight why MPLA induces immune activation without provoking toxicity in human and clarifies why MPLA can be used as activating compound for clinically applicable immuno-activatory cellular products grown in serum-free regimens.




Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- MPLA:
-
Monophosphoryl lipid A
- LBP:
-
LPS-binding protein
- MPLAs:
-
MPLA derived from S. minnesota re595
- LPSs:
-
LPS derived from S. typhimurium
- MPLAe:
-
MPLA derived from E. coli r515
- LPSe:
-
LPS derived from E. coli O111B4
- RANTES:
-
Chemokine CCL5
References
Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6:133–40.
Schwarz TF. Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine. Cervarix Adv Ther. 2009;26:983–98.
Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–40.
Garcon N, Wettendorff M, van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther. 2011;11:667–77.
Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71:2498–507.
ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25:7145–52.
McAleer JP, Vella AT. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol. 2010;31:429–35.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–55.
Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–9.
Hoebe K, Du X, Goode J, Mann N, Beutler B. Lps2: a new locus required for responses to lipopolysaccharide, revealed by germline mutagenesis and phenotypic screening. J Endotoxin Res. 2003;9:250–5.
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–3.
Kolanowski ST, Dieker MC, Lissenberg-Thunnissen SN, van Schijndel GM, van Ham SM, ten Brinke A. TLR4-mediated pro-inflammatory dendritic cell differentiation in humans requires the combined action of MyD88 and TRIF. Innate Immun. 2013;20:423–30.
Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32:116–33.
Pugin J, Schurer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–8.
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3.
Ulevitch RJ. Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv Immunol. 1993;53:267–89.
Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–51.
Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82.
Bingle CD, Craven CJ. Meet the relatives: a family of BPI- and LBP-related proteins. Trends Immunol. 2004;25:53–5.
Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. 2011;39:989–93.
Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204.
Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–8.
Tobias PS, Soldau K, Kline L, Lee JD, Kato K, Martin TP, Ulevitch RJ. Cross-linking of lipopolysaccharide (LPS) to CD14 on THP-1 cells mediated by LPS-binding protein. J Immunol. 1993;150:3011–21.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Casella CR, Mitchell TC. Inefficient TLR4/MD-2 Heterotetramerization by Monophosphoryl Lipid A. PLoS One. 2013;8:e62622.
Maeshima N, Fernandez RC. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell Infect Microbiol. 2013;3:3.
Tsukamoto H, Fukudome K, Takao S, Tsuneyoshi N, Kimoto M. Lipopolysaccharide-binding protein-mediated Toll-like receptor 4 dimerization enables rapid signal transduction against lipopolysaccharide stimulation on membrane-associated CD14-expressing cells. Int Immunol. 2010;22:271–80.
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–5.
Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–9.
Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–80.
Roy S, Karmakar M, Pearlman E. CD14 mediates Toll-like receptor 4 (TLR4) endocytosis and spleen tyrosine kinase (Syk) and interferon regulatory transcription factor 3 (IRF3) activation in epithelial cells and impairs neutrophil infiltration and Pseudomonas aeruginosa killing in vivo. J Biol Chem. 2014;289:1174–82.
Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–70.
Lloyd-Jones KL, Kelly MM, Kubes P. Varying importance of soluble and membrane CD14 in endothelial detection of lipopolysaccharide. J Immunol. 2008;181:1446–53.
Gangloff M. Different dimerisation mode for TLR4 upon endosomal acidification? Trends Biochem Sci. 2012;37:92–8.
Tanimura N, Saitoh SI, Ohto U, Akashi-Takamura S, Fujimoto Y, Fukase K, Shimizu T, Miyake K. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int Immunol. 2014;16:307–14.
Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8.
Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32.
Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–4.
ten Brinke A, van Schijndel G, Visser R, de Gruijl TD, Zwaginga JJ, van Ham SM. Monophosphoryl lipid A plus IFNgamma maturation of dendritic cells induces antigen-specific CD8 + cytotoxic T cells with high cytolytic potential. Cancer Immunol Immunother. 2010;59:1185–95.
Gangloff SC, Zahringer U, Blondin C, Guenounou M, Silver J, Goyert SM. Influence of CD14 on ligand interactions between lipopolysaccharide and its receptor complex. J Immunol. 2005;175:3940–5.
Wright SD. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8.
Kim D, Kim JY. Anti-CD14 antibody reduces LPS responsiveness via TLR4 internalization in human monocytes. Mol Immunol. 2013;57:210–5.
Kolb JP, Casella CR, SenGupta S, Chilton PM, Mitchell TC. Type I interferon signaling contributes to the bias that Toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci Signal. 2014;7:ra108.
Triantafilou M, Lepper PM, Olden R, Dias IS, Triantafilou K. Location, location, location: is membrane partitioning everything when it comes to innate immune activation? Mediat Inflamm. 2011;2011:186093.
Resman N, Oblak A, Gioannini TL, Weiss JP, Jerala R. Tetraacylated lipid A and paclitaxel-selective activation of TLR4/MD-2 conferred through hydrophobic interactions. J Immunol. 2014;192:1887–95.
Beamer LJ, Carroll SF, Eisenberg D. The BPI/LBP family of proteins: a structural analysis of conserved regions. Protein Sci. 1998;7:906–14.
Beamer LJ, Carroll SF, Eisenberg D. The three-dimensional structure of human bactericidal/permeability-increasing protein: implications for understanding protein-lipopolysaccharide interactions. Biochem Pharmacol. 1999;57:225–9.
Kelley SL, Lukk T, Nair SK, Tapping RI. The crystal structure of human soluble CD14 reveals a bent solenoid with a hydrophobic amino-terminal pocket. J Immunol. 2013;190:1304–11.
Jahr TG, Sundan A, Lichenstein HS, Espevik T. Influence of CD14, LBP and BPI in the monocyte response to LPS of different polysaccharide chain length. Scand J Immunol. 1995;42:119–27.
Anas AA, Hovius JWR, van’t Veer C, van der Poll T, de Vos AF. Role of CD14 in a mouse model of acute lung inflammation induced by different lipopolysaccharide chemotypes. PLoS One. 2010;5:e10183.
Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353–63.
Kitchens RL, Munford RS. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–10.
Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi. 529. Expert Rev Vaccines. 2003;2:219–29.
Watanabe S, Kumazawa Y, Inoue J. Liposomal lipopolysaccharide initiates TRIF-dependent signaling pathway independent of CD14. PLoS One. 2013;8:e60078_1–7.
Acknowledgments
We thank Gijs van Schijndel and Miranda Dieker for technical assistance. We thank Prof. dr. van der Schoot, Sanquin Blood Supply for provision of mouse anti-human CD14.22 antibodies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a grant from the Joghem van Loghem foundation.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Electronic supplementary material
Below is the link to the electronic supplementary material.
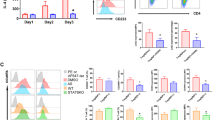
Fig. S1
MPLA does not require LBP to induce cytokine production. (A,B) IL-6 production by DCs stimulated with a titration of LBP in serum-free IMDM in presence of (A) 10 ng/ml LPSe, one representative experiment out of 6 is shown, or (B) 10 μg/ml MPLAe, a representative experiment (n = 7). (C,D) TNF-α (C) and IL-6 (D) production by DCs stimulated with indicated concentrations of MPLAs in presence or absence of LBP. Cytokine production is shown relative to stimulation with comparable amounts of MPLAs in absence of LBP, n = 8. For statistical analysis a paired t test was performed (EPS 92 kb)
Fig. S2
RANTES production is solely TRIF dependent in human moDCs. To verify that production of chemokine RANTES was TRIF dependent, MyD88 or TRIF was down-regulated using siRNA prior to stimulation of iDCs with MPLAe. To down-regulate MyD88 of TRIF iDCs were electroporated with control siRNA (siC) or specific siRNA targeting MyD88 (siM) or TRIF (siT). Two days after electroporation iDCs were stimulated with MPLAe. Production of TNF-α and IL-6 was lower in MyD88- and TRIF down-regulated DCs compared to control siRNA-treated DCs (A, B) while production of RANTES was only decreased in TRIF down-regulated DCs, but not MyD88 down-regulated DCs (C). Concentration of cytokines TNF-α (A), IL-6 (B) or chemokine RANTES (C) was determined in supernatant harvested 24 h after stimulation with MPLA, siMyD88, n = 17, siTRIF n = 8, a paired t test was performed for statistical analysis, values were compared to siC condition *: p < 0.05 (EPS 117 kb)
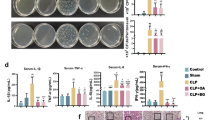
Fig. S3
CD14 is not required for MPLAe-mediated cytokine production. (A-C) Cytokine production by DCs stimulated with LPSe in the presence of control antibodies (CTRL, grey lines) or CD14-blocking antibodies (a-CD14, black lines). LPSe-stimulated production of TNF-α (A), IL-6 (B), a representative experiment (n = 7). (C) LPSe responsiveness by a-CD14 treatment is quantified as described in materials & methods, n = 4. (D,E) MPLAe-stimulated production of TNF-α (D) or IL-6 (E), one representative experiment out of 9 is shown. (F,G) MPLAe-stimulated production of TNF-α (F), IL-6 (G), as a % of CTRL condition n = 6. (C, F, G) A one sample t test was performed for statistical analysis. *: p < 0.05, **: p < 0.01 (EPS 145 kb)
Rights and permissions
About this article
Cite this article
Kolanowski, S.T.H.M., Lissenberg-Thunnissen, S.N., Emal, D. et al. Monophosphoryl lipid A-induced pro-inflammatory cytokine expression does not require CD14 in primary human dendritic cells. Inflamm. Res. 65, 449–458 (2016). https://doi.org/10.1007/s00011-016-0927-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0927-0