Abstract
Aim and objective
Cardiac fibrosis is an important pathological feature of cardiac remodeling in heart diseases. Methyl-CpG-binding protein 2 (MeCP2) is a transcription inhibitor, and plays a key role in the fibrotic diseases. However, the precise role of MeCP2 in cardiac fibrosis remains unclear. α-tubulin plays an essential role in cell function, whereby the acetylation state of α-Tubulin dictates the efficiency of cell proliferation and differentiation. This study was undertaken to investigate that MeCP2 dynamics affect the acetylation state of α-tubulin in the cardiac fibrosis.
Methods
Forty adult male Sprague–Dawley (SD) rats were randomly divided into two groups, cardiac fibrosis was produced by common ISO. Cardiac fibroblasts (CFs) were harvested from SD neonate rats and cultured. The expression of HDAC6, MeCP2, α-SMA, collagen I was measured by western blotting and qRT-PCR. siRNA of HDAC6 and MeCP2 effect the proliferation of cardiac fibroblasts, and affect the acetylation state of α-tubulin.
Results
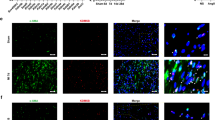
We have found the acetylation state of α-tubulin in cardiac fibroblasts as well as cardiac tissue from a ISO-induced rat cardiac fibrosis model and observed a reduction in acetylated α-tubulin and an increase in the α-tubulin-specific deacetylase, histone deacetylase 6 (HDAC6). Furthermore, we have shown that treatment of cardiac fibroblasts with HDAC6 inhibitor Tubastatin A and HDAC6-siRNA can restore α-tubulin acetylation levels. In addition, treatment of cardiac fibroblasts with MeCP2-siRNA blocked cell proliferation. Knockdown of MeCP2 suppresses HDAC6 expression in activated cardiac fibroblasts but increases the acetylation of α-tubulin.
Conclusions
We demonstrated that MeCP2 may negatively control the acetylation of α-tubulin through HDAC6 in cardiac fibroblast proliferation and fibrosis. This study indicated that MeCP2 could be a potentially new therapeutic option for cardiac fibrosis.






Similar content being viewed by others
Abbreviations
- HDAC6:
-
Histone deacetylase 6
- HATs:
-
Histone acetyltransferases
- ECM:
-
Extracellular matrix
- MeCP2:
-
Methyl-CpG-binding protein 2
- α-SMA:
-
Smooth muscle α-action
- TGF-β1:
-
Transforming growth factor-β1
- Col1A1:
-
Type I collagen
- MBD:
-
Methyl-CpG-binding domain
- TRD:
-
Transcriptional repression domain
- CFs:
-
Cardiac fibroblasts
- ISO:
-
Isoprenaline
References
Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. European journal of heart failure 2014.
Li Q, Liu X, Wei J. Ageing related periostin expression increase from cardiac fibroblasts promotes cardiomyocytes senescent. Biochem Biophys Res Commun. 2014;452:497–502.
Abe Y. Myocardial fibrosis of the left ventricular posterior wall can be a target for early detection of cardiac involvement in patients with Duchenne muscular dystrophy. Journal of cardiology 2015.
Lindner D, Zietsch C, Tank J, Sossalla S, Fluschnik N, Hinrichs S, et al. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res Cardiol. 2014;109:428.
Guo JL, Yu Y, Jia YY, Ma YZ, Zhang BY, Liu PQ, et al. Transient receptor potential melastatin 7 (TRPM7) contributes to H2O2-induced cardiac fibrosis via mediating Ca(2+) influx and extracellular signal-regulated kinase 1/2 (ERK1/2) activation in cardiac fibroblasts. J Pharmacol Sci. 2014;125:184–92.
Wang H, Zhao Z, Lin M, Groban L. Activation of GPR30 inhibits cardiac fibroblast proliferation. Molecular and cellular biochemistry 2015.
Wu L, Ong S, Talor MV, Barin JG, Baldeviano GC, Kass DA, et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J Exp Med. 2014;211:1449–64.
Feridooni T, Hotchkiss A, Remley-Carr S, Saga Y, Pasumarthi KB. Cardiomyocyte specific ablation of p53 is not sufficient to block doxorubicin induced cardiac fibrosis and associated cytoskeletal changes. PLoS One. 2011;6:e22801.
Roll-Mecak A. Intrinsically disordered tubulin tails: complex tuners of microtubule functions? Seminars in cell and developmental biology 2014.
Husain M, Cheung CY. Histone deacetylase 6 inhibits influenza a virus release by downregulating the trafficking of viral components to the plasma membrane via its substrate, acetylated microtubules. J Virol. 2014;88:11229–39.
Matsushita T, Fujihara A, Royall L, Kagiwada S, Kosaka M, Araki M. Immediate differentiation of neuronal cells from stem/progenitor-like cells in the avian iris tissues. Exp Eye Res. 2014;123:16–26.
Sunami Y, Araki M, Hironaka Y, Morishita S, Kobayashi M, Liew EL, et al. Inhibition of the NAD-dependent protein deacetylase SIRT2 induces granulocytic differentiation in human leukemia cells. PLoS One. 2013;8:e57633.
Tao H, Yang JJ, Shi KH, Deng ZY, Li J. DNA methylation in cardiac fibrosis: new advances and perspectives. Toxicology. 2014;323:125–9.
Tao H, Shi KH, Yang JJ, Huang C, Zhan HY, Li J. Histone deacetylases in cardiac fibrosis: current perspectives for therapy. Cell Signal. 2014;26:521–7.
Nagai T, Ikeda M, Chiba S, Kanno S, Mizuno K. Furry promotes acetylation of microtubules in the mitotic spindle by inhibition of SIRT2 tubulin deacetylase. J Cell Sci. 2013;126:4369–80.
Kang SH, Seok YM, Song MJ, Lee HA, Kurz T, Kim I. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol Pharmacol. 2015;87:782–91.
Kee HJ, Bae EH, Park S, Lee KE, Suh SH, Kim SW, et al. HDAC inhibition suppresses cardiac hypertrophy and fibrosis in DOCA-salt hypertensive rats via regulation of HDAC6/HDAC8 enzyme activity. Kidney Blood Press Res. 2013;37:229–39.
Tang G, Wong JC, Zhang W, Wang Z, Zhang N, Peng Z, et al. Identification of a novel aminotetralin class of HDAC6 and HDAC8 selective inhibitors. J Med Chem. 2014;57:8026–34.
Tala, Sun X, Chen J, Zhang L, Liu N, Zhou J, et al. Microtubule stabilization by Mdp3 is partially attributed to its modulation of HDAC6 in addition to its association with tubulin and microtubules. PloS One 2014; 9:e90932.
Gold WA, Lacina TA, Cantrill LC, Christodoulou J. MeCP2 deficiency is associated with reduced levels of tubulin acetylation and can be restored using HDAC6 inhibitors. J Mol Med (Berl) 2014.
Meng G, Lv Y, Dai H, Zhang X, Guo QN. Epigenetic silencing of methyl-CpG-binding protein 2 gene affects proliferation, invasion, migration, and apoptosis of human osteosarcoma cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014.
Heckman LD, Chahrour MH, Zoghbi HY. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. eLife 2014; 3.
Kou CY, Lau SL, Au KW, Leung PY, Chim SS, Fung KP, et al. Epigenetic regulation of neonatal cardiomyocytes differentiation. Biochem Biophys Res Commun. 2010;400:278–83.
Alvarez-Saavedra M, Carrasco L, Sura-Trueba S, Demarchi Aiello V, Walz K, Neto JX, et al. Elevated expression of MeCP2 in cardiac and skeletal tissues is detrimental for normal development. Hum Mol Genet. 2010;19:2177–90.
Kohyama J, Kojima T, Takatsuka E, Yamashita T, Namiki J, Hsieh J, et al. Epigenetic regulation of neural cell differentiation plasticity in the adult mammalian brain. Proc Natl Acad Sci USA. 2008;105:18012–7.
Cheng F, Lienlaf M, Perez-Villarroel P, Wang HW, Lee C, Woan K, et al. Divergent roles of histone deacetylase 6 (HDAC6) and histone deacetylase 11 (HDAC11) on the transcriptional regulation of IL10 in antigen presenting cells. Mol Immunol. 2014;60:44–53.
Acknowledgments
This project was supported by Anhui Provincial Natural Science Foundation (1408085MH175, 1308085MH117).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Responsible Editor: Liwu Li.
Rights and permissions
About this article
Cite this article
Tao, H., Yang, JJ., Shi, KH. et al. Epigenetic factors MeCP2 and HDAC6 control α-tubulin acetylation in cardiac fibroblast proliferation and fibrosis. Inflamm. Res. 65, 415–426 (2016). https://doi.org/10.1007/s00011-016-0925-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0925-2