Abstract
Objective
To determine whether inflammation following anterior cruciate ligament (ACL) reconstruction leads to long-term pathological changes in the infrapatellar fat pad (IPFP or Hoffa’s fat pad) which could compromise the integrity of the knee joint.
Materials and methods
Sixteen mature sheep underwent anatomic idealized ACL reconstruction surgery (ACL-R) and were sacrificed at 2 weeks (n = 9) and 20 weeks (n = 7) post-ACL-R. Five additional animals served as unoperated controls. A histological grading protocol was developed to quantify the changes in the IPFP post-injury. mRNA expression levels for key markers of inflammation, angiogenesis and tissue regeneration were assessed by qPCR.
Results
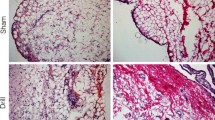
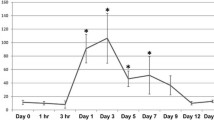
The IPFP exhibited altered cellularity and fibrosis at 2 and 20 weeks post-ACL-R. Immunohistochemistry detected macrophage-like cells in the IPFP which were increased at 20 weeks. Specific pro-inflammatory cytokines and IPFP specific adipokines exhibited changes indicating early inflammation mediated alterations. Elevations in CD105 mRNA levels at 2 weeks corroborated the increases in neovascularization observed in the IPFP following injury.
Conclusions
Sustained long-term pathological changes stemming from inflammation are present in IPFP tissue after ACL-R surgery and may compromise the long-term integrity of the knee joint.






Similar content being viewed by others
References
Galllagher J, Tierney P, Murray P, O’Brien M. The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):268–72.
Jacobson JA, Lenchik L, Ruhoy MK, Schweitzer ME, Resnick D. MR imaging of the infrapatellar fat pad of Hoffa. Radiographics. 1997;17:675–91.
Ballegaard C, Riis RG, Bliddal H, Christensen R, Henriksen M, Bartels EM, Lohmander LS, Hunter DJ, Bouert R, Boesen M. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthr Cartil. 2014;22(7):933–40.
Clockaerts S, Bastiaansen-Jenniskens YM, Van Osch GV, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue. Osteoarthr Cartil. 2010;18(7):876–82.
Neuman P, Kostogiannis I, Fridén T, et al. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury—a prospective cohort study. Osteoarthr Cartil. 2009;17(3):284–90.
Gillquist J, Messner K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999;27(3):143–56.
Daniel DM, Stone ML, Dobson BE, et al. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–44.
Heard BJ, Achari Y, Chung M, et al. Early joint tissue changes are highly correlated with a set of inflammatory and degradative synovial biomarkers after ACL autograft and its sham surgery in an ovine model. J Orthop Res. 2011;29(8):1185–92.
Heard BJ, Solbak NM, Achari Y, et al. Changes of early post-traumatic osteoarthritis in an ovine model of simulated ACL reconstruction are associated with transient acute post-injury synovial inflammation and tissue catabolism. Osteoarthr Cartil. 2013;21(12):1942–9.
Frayn KN, Karpe F, Fielding BA, et al. Integrative physiology of human adipose tissue. Int J Obes Metab Disord. 2003;27(8):875–88.
Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62(2):108–12.
Abreu MR, Chung CB, Trudell D, Resnick D. Hoffa’s fat pad injuries and their relationship with anterior cruciate ligament tears: new observations based on MR imaging in patients and MR imaging and anatomic correlation in cadavers. Skelet Radiol. 2008;37(4):301–6.
Drez DJ, DeLee J, Holden JP, et al. Anterior cruciate ligament reconstruction using bone- patellar tendon-bone allografts. A biological and biomechanical evaluation in goats. Am J Sports Med. 1991;19(3):256–63.
Adams ME, Billingham ME, Muir H. The glycosaminoglycans in menisci in experimental and natural osteoarthritis. Arthritis Rheum. 1983;26(1):69–76.
Cummings JF, Grood ES, Levy MS, et al. The effects of graft width and graft laxity on the outcome of caprine anterior cruciate ligament reconstruction. J Orthop Res. 2002;20(2):338–45.
Reno C, Marchuk L, Sciore P, et al. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22(6):1082–6.
Lemor A, Mielenz M, Altmann M, et al. mRNA abundance of adiponectin and its receptors, leptin and visfatin and of G-protein coupled receptor 41 in five different fat depots from sheep. J Anim Physiol Anim Nutr. 2010;94(5):96–101.
Remst DF, Blom AB, Vitters EL, Bank RA, van den Berg WB, Blaney Davidson EN, van der Kraan PM. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 2014;66(3):647–56.
Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res. 2014;2014:726861 Epub 2014 Jan 19.
Warrington K, Hillarby MC, Li C, Letarte M, Kumar S. Functional role of CD105 in TGF-beta1 signalling in murine and human endothelial cells. Anticancer Res. 2005;25(3B):1851–64 PubMed PMID: 16158917.
Jantsch J, Binger KJ, Müller DN, Titze J. Macrophages in homeostatic immune function. Front Physiol. 2014;5:146. doi:10.3389/fphys.2014.00146. eCollection 2014.
Stålman A, Bring D, Ackermann PW. Chemokine expression of CCL2, CCL3, CCL5 and CXCL10 during early inflammatory tendon healing precedes nerve regeneration: an immuno-histochemical study in the rat. Knee Surg Sports Traumatol Arthrosc. 2014. [Epub ahead of print].
Ackermann PW, Salo PT, Hart DA. Neuronal pathways in tendon healing. Front Biosci (Landmark Ed). 2009;14:5165–87. Review. PubMed PMID: 19482611.
Ackermann PW, Franklin SL, Dean BJ, Carr AJ, Salo PT, Hart DA. Neuronal pathways in tendon healing and tendinopathy–update. Front Biosci (Landmark Ed). 2014;1(19):1251–78.
O’Brien EJ, Beveridge JE, Huebner KD, et al. Osteoarthritis develops in the operated joint of an ovine model following ACL reconstruction with immediate anatomic reattachment of the native ACL. J Orthop Res. 2013;31(1):35–43.
Turhan E, Doral MN, Atay AO, Demirel M. A giant extrasynovial osteochondroma in the infrapatellar fat pad: end stage Hoffa’s disease. Arch Orthop Trauma Surg. 2008;128(5):515–9.
Sun K, Tordjman J, Cle’ment K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–7.
Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Investig Dermatol. 2007;127(5):1009–17.
Clements KM, Ball AD, Jones HB, Brinckmann S, Read SJ, Murray F. Cellular and histopathological changes in the infrapatellar fat pad in the monoiodoacetate model of osteoarthritis pain. Osteoarthr Cartil. 2009;17(6):805–12.
Murakami S, Muneta T, Furuya K, Saito I, Miyasaka N, Yamamoto H. Immunohistologic analysis of synovium in infrapatellar fat pad after anterior cruciate ligament injury. Am J Sports Med. 1995;23(6):763–8.
Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11:203.
Frank CB, Shrive NG, Lo IKY, Hart DA. Form and function of tendon and ligament. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic basic science: biology and biomechanics of the musculoskeletal system. Rosemont: American Academy of Orthopaedic Surgeons; 2007.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ [review]. Arthritis Rheum. 2012;64:1697–707.
Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–7.
Gierman LM, Wopereis S, van El B, Verheij ER, der Vat Werff-van BJ, Bastiaansen-Jenniskens YM, van Osch GJ, Kloppenburg M, Stojanovic-Susulic V, Huizinga TW, Zuurmond AM. Metabolic profiling reveals differences in concentrations of oxylipins and fatty acids secreted by the infrapatellar fat pad of donors with end-stage osteoarthritis and normal donors. Arthritis Rheum. 2013;65(10):2606–14.
Iwata M, Ochi H, Hara Y, et al. 2013 Initial responses of articular tissues in a murine high fat diet induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PLoS One. 2013;8(4):e60706.
Clockaerts S, Bastiaansen-Jenniskens YM, Feijt C, et al. Cytokine production by infrapatellar fat pad can be stimulated by interleukin 1β and inhibited by peroxisome proliferator activated receptor α agonist. Ann Rheum Dis. 2012;71(6):1012–8.
Van Beeck A, Clockaerts S, Somville J, et al. Does infrapatellar fat pad resection in total knee arthroplasty impair clinical outcome? A systematic review. The Knee. 2013;20(4):226–31.
Pappa CA, Alexandrakis MG, Boula A, Psarakis FE, Kolovou A, Bantouna V, Stavroulaki E, Tsirakis G. Emerging roles of endoglin/CD105 and angiogenic cytokines for disease development and progression in multiple myeloma patients. Hematol Oncol. 2013;31(4):201–5.
Lim J, Hotchin NA. Signalling mechanisms of the leukocyte integrin alphaM-beta2: current and future perspectives. Biol Cell. 2012;104(11):631–40.
Blom AB, van Lent PE, Holthuysen AE, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr Cartil. 2004;12(8):627–35.
Van Lent PL, Blom AB, van der Kraan P, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50(1):103–11.
Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1488–95.
Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–83.
Tanaka N, Sakahashi H, Sato E, et al. Influence of the infrapatellar fat pad resection in a synovectomy during total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplast. 2003;18(7):897–902.
Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71(2):288–94.
Felimban R, Ye K, Traianedes K, Di Bella C, Crook J, Wallace GG, Quigley A, Choong PF, Myers DE. Differentiation of stem cells from human infrapatellar fat pad: characterization of cells undergoing chondrogenesis. Tissue Eng Part A. 2014;20(15–16):2213–23.
Acknowledgments
The authors gratefully acknowledge the financial support provided for these studies through research grants from the Canadian Institutes for Health Research (MOP-9858 & IMH-120277) (CBF and NGS),The Arthritis Society (SOG-12-04), and an Alberta Innovates Health Solutions Team Grant in Osteoarthritis ((1) 20080170 (2) 200700596) (CBF, NGS and DAH) and Bridge Funding from the Cumming School of Medicine (DAH & NGS). NMS was supported through a studentship from the Alberta Innovates Health Solutions Team Grant in Osteoarthritis. BJH was supported through fellowships from The Arthritis Society and a University of Calgary Queen Elizabeth II Scholarship. NGS is a Killam Memorial Professor and CBF was a McCaig Professor at the McCaig Institute for Bone and Joint Health. The authors thank Leslie Jacques for excellent technical expertise.
Funding
The funds for these studies were obtained from public granting agencies such as Canadian Institutes for Health Research, The Arthritis Society, and an Alberta Innovates Health Solutions Team Grant in Osteoarthritis and Bridge Funding from Cumming School of Medicine. The authors state that there were no private sponsors for this study.
Conflict of interest
The authors declare that there are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jason J. McDougall.
Cyril B. Frank—Deceased.
Rights and permissions
About this article
Cite this article
Solbak, N.M., Heard, B.J., Achari, Y. et al. Alterations in Hoffa’s fat pad induced by an inflammatory response following idealized anterior cruciate ligament surgery. Inflamm. Res. 64, 615–626 (2015). https://doi.org/10.1007/s00011-015-0840-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0840-y